Sulfonyl fluoride compound and application thereof
A sulfonyl fluoride-based compound technology, applied in the field of sulfonyl fluoride-based compounds, can solve the problems of unfavorable clinical transformation and harsh labeling conditions, and achieve the effects of high yield, high affinity and simple labeling conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
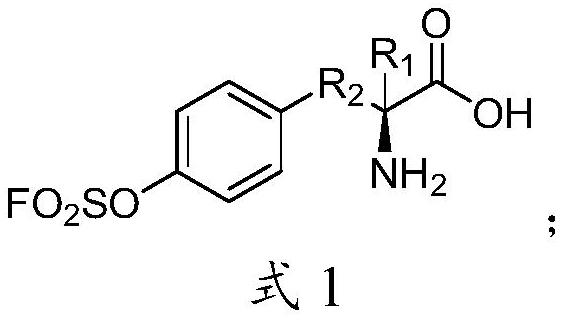
[0033] Example 1 (S)-2-amino-3-(4-((fluorosulfonyl)oxy)phenyl)propionic acid
[0034] The structural formula is as follows:
[0035]
[0036] The synthetic route is as follows:
[0037]
[0038] (1) Synthesis of (S)-2-(((tert-butoxycarbonyl)amino)tert-butyl-3-(4-((fluorosulfonyl)oxy)phenyl)propionate
[0039] Compound (S)-2-amino-3-(4-((fluorosulfonyl)oxy)phenyl)propionic acid (0.5g, 1.48mmol) was dissolved in 20mL of dichloromethane, in ice water, and then Add triethylamine (0.59mL, 5.93mmol), SO 2 A balloon of ClF gas was inserted under the liquid and reacted overnight. Afterwards, the reaction solution was added to water, dried over anhydrous sodium sulfate, concentrated, and column chromatographed to obtain the product (0.36 g, 58.4%). 1H NMR (300MHz, CDCl3) δ7.30(s, 4H), 5.09(d, J=4.9Hz, 1H), 4.47(d, J=5.5Hz, 1H), 3.09(s, 2H), 1.42(d ,J=7.2Hz,18H).13C NMR(75MHz,CDCl3)δ170.46,155.03,148.97,137.66,131.43,120.67,82.52,79.72,77.44,77.02,76.59,54.67,38.13,28.25,28Sc...
example 2
[0042] Example 2 (2S, 4S)-2,5-diamino-4-(4-((fluorosulfonyl)oxy)benzyl)-5-oxopentanoic acid
[0043] The structural formula is as follows:
[0044]
[0045] The synthetic route is as follows:
[0046]
[0047] (1) Synthesis of tert-butyl (2S, 4S)-2-(((tert-butoxycarbonyl)amino)-4-(4-((fluorosulfonyl)oxy)benzyl)-5-oxo-5 -((2,4,5-Trimethoxybenzyl)amino)pentanoate
[0048]The compound (2S, 4S)-2-(((tert-butoxycarbonyl)amino)-4-(4-hydroxybenzyl)-5-oxo-5-((2,4,5-trimethoxybenzyl Base) amino) tert-butyl valerate (0.2g, 0.34mmol) was dissolved in 20mL of dichloromethane, as in ice water, then added triethylamine (0.13mL, 1.3mmol), SO 2 A balloon of ClF gas was inserted under the liquid and reacted overnight. Afterwards, the reaction solution was added to water, dried over anhydrous sodium sulfate, concentrated, and subjected to column chromatography to obtain the product (0.13 g, 56.2%). 1 H NMR (300MHz, CDCl3) δ7.09(d, J=6.1Hz, 2H), 6.92(d, J=6.6Hz, 2H), 5.94(s, 2H), 5.19...
example 3
[0051] Example 3 2-amino-3-(4-((fluorosulfonyl)oxy)phenyl)-2-methylpropionic acid
[0052] The structural formula is as follows:
[0053]
[0054] The synthetic route is as follows:
[0055]
[0056] (1) Synthesis of tert-butyl 2-((tert-butoxycarbonyl)amino)-3-(4-((fluorosulfonyl)oxy)phenyl)-2-methylpropionate
[0057] The compound 2-((tert-butoxycarbonyl)amino)-3-(4-hydroxyphenyl)-2-methylpropanoic acid tert-butyl ester (0.2 g, 0.56 mmol) was dissolved in 20 mL of dichloromethane, as After adding triethylamine (2.2mL, 2.27mmol) to ice water, the SO 2 A balloon of ClF gas was inserted under the liquid and reacted overnight. Afterwards, the reaction solution was added to water, dried over anhydrous sodium sulfate, concentrated, and subjected to column chromatography to obtain the product (0.15 g, 63.4%). HRMS calcd for; C19H28FNO7S 433.1571found, 434.1689[M+H] + .
[0058] (2) Synthesis of 2-amino-3-(4-((fluorosulfonyl)oxy)phenyl)-2-methylpropionic acid
[0059] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com