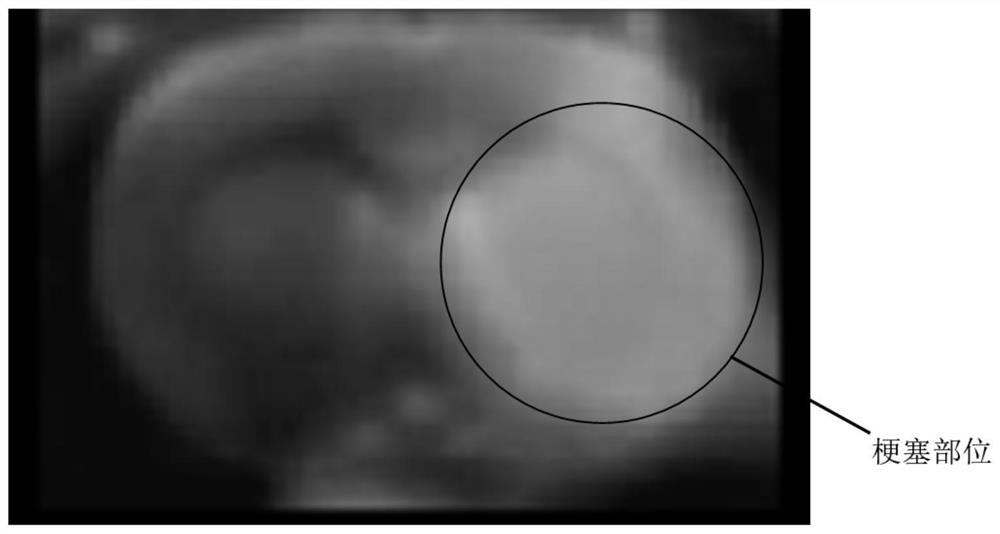
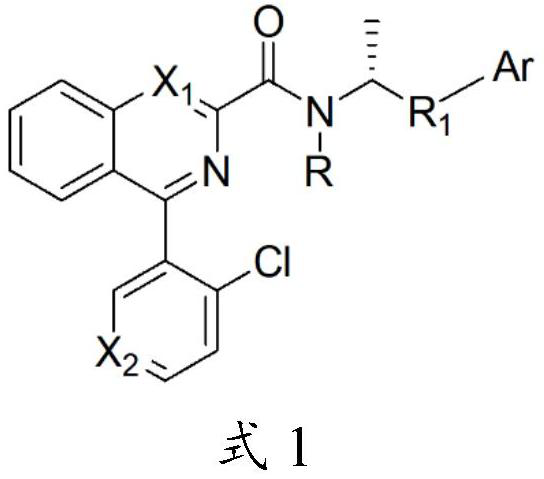
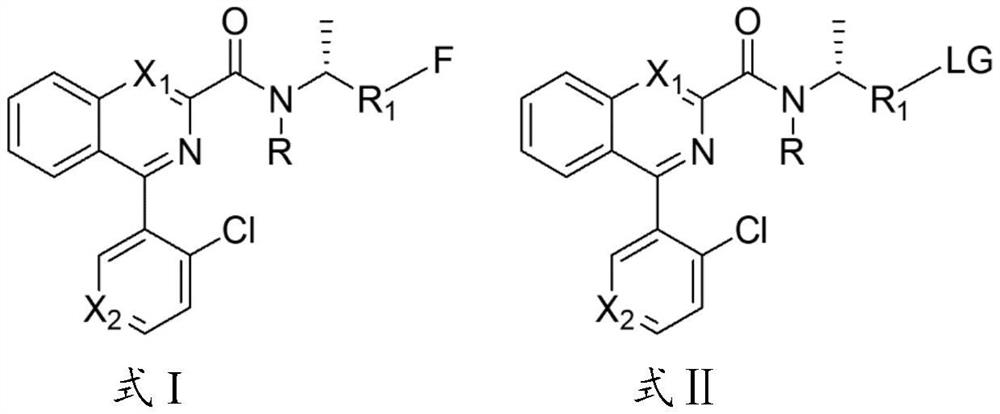
4-(2-chloroaryl) quinazoline-2-amide derivative and application thereof
A technology of amide derivatives and derivatives, applied in the fields of radiopharmaceutical chemistry and nuclear medicine, can solve the problems of complex labeling conditions, short half-life of ER-176, and inability to transport long distances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0029] The synthesis of example 1 (R)-4-(2-chlorophenyl)-N-(4-fluorobut-2-yl)-N-methylquinazoline-2-carboxamide, its structural formula is as follows:
[0030]
[0031] The synthetic route is as follows:
[0032]
[0033] (1) Preparation of (R)-4-(2-chlorophenyl)-N-(4-hydroxybut-2-yl)quinazoline-2-carboxamide
[0034] N 2 Under protection, 4-(2-chlorophenyl)quinazoline-2-carboxylic acid (0.31g, 1.07mmol) was dissolved in anhydrous dichloromethane (20mL), and EDCI (1.02g, 5.35mmol) was added successively , HOBt (0.04g, 0.22mmol) and TEA (0.54g, 5.35mmol), add (R)-3-aminobutyl-1-ol (0.12g, 1.29mmol), stir well, and react overnight at room temperature. After stopping the reaction, the reaction solution was washed with water and saturated brine successively, the organic layer was dried and rotary evaporated, and separated by a column (DCM / MeOH=95 / 5) to obtain 0.23 g of light yellow oil with a yield of 60.7%. 1 H NMR (300MHz, CDCl 3 )δ8.35(d, J=8.5Hz, 1H), 8.22(d, J=8.4Hz...
example 2
[0044] Example 2 (R)-4-(2-chlorophenyl)-N-(4-fluorobut-2-yl)-N-(methyl-d 3 ) the synthesis of quinazoline-2-carboxamide, its structural formula is as follows:
[0045]
[0046] The synthetic route is as follows:
[0047]
[0048] (1) Preparation of 4-(2-chlorophenyl)-N-(methyl-d 3 )-N-((2R)-4-((tetrahydro-2H-pyran-2-yl)oxy)butan-2-yl)quinazoline-2-carboxamide
[0049] Under ice-cooling, the starting material (0.22g, 0.50mmol) was dissolved in anhydrous DMF (15mL), NaH (0.04mg, 1.50mmol) and deuteroiodomethane (0.22g, 1.50mmol) were added, and ice-bathed to Reaction at room temperature, the raw material disappeared after 0.5h. The reaction was quenched with ice water, and saturated brine (30mL x 5) was added to wash the DCM layer, the organic phase was dried and rotary evaporated, and column separation gave 0.21g of a colorless oil, with a yield of 92.2%. MS ES + (m / z):479.2220[M+Na] +
[0050] (2) Preparation of (R)-4-(2-chlorophenyl)-N-(4-hydroxybut-2-yl)-N-(meth...
example 3
[0056] Example 3 Synthesis of 4-(2-chlorophenyl)-N-((2R)-4-fluorobut-2-yl-4-d)-N-methylquinazoline-2-carboxamide, its structural formula as follows:
[0057]
[0058] The synthetic route is as follows:
[0059]
[0060] (1) Preparation of (R)-4-(2-chlorophenyl)-N-methyl-N-(4-oxobut-2-yl)quinazoline-2-carboxamide
[0061] The starting material (0.06 g, 0.17 mmol) was dissolved in anhydrous DCM (20 mL) in an ice bath, and Dess-Martin oxidant (0.22 g, 0.51 mmol) and NaHCO were added 3 (0.10g, 1.19mmol), reacted at 0°C, monitored by TLC, and the reaction was complete after 1.5h. After stopping the reaction, add anhydrous DCM (50mL), add sodium thiosulfate aqueous solution (20mL, 1mol / L) and saturated NaHCO 3 Aqueous solution (20 mL), stirred for 10 min, the organic layer was separated, dried and rotary evaporated to obtain 0.05 g of a colorless oily product with a yield of 79.4%. MS ES + (m / z):368.1150[M+H] +
[0062](2) Preparation of (R)-4-(2-chlorophenyl)-N-methyl-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com