Polyhydroxy compound as well as preparation method and application thereof
A polyol compound and reaction technology, applied in organic chemistry, electrochemical generators, structural parts, etc., can solve the problems of easily damaged structure and poor stability, and achieve enhanced conductivity, good stability, and increased size Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
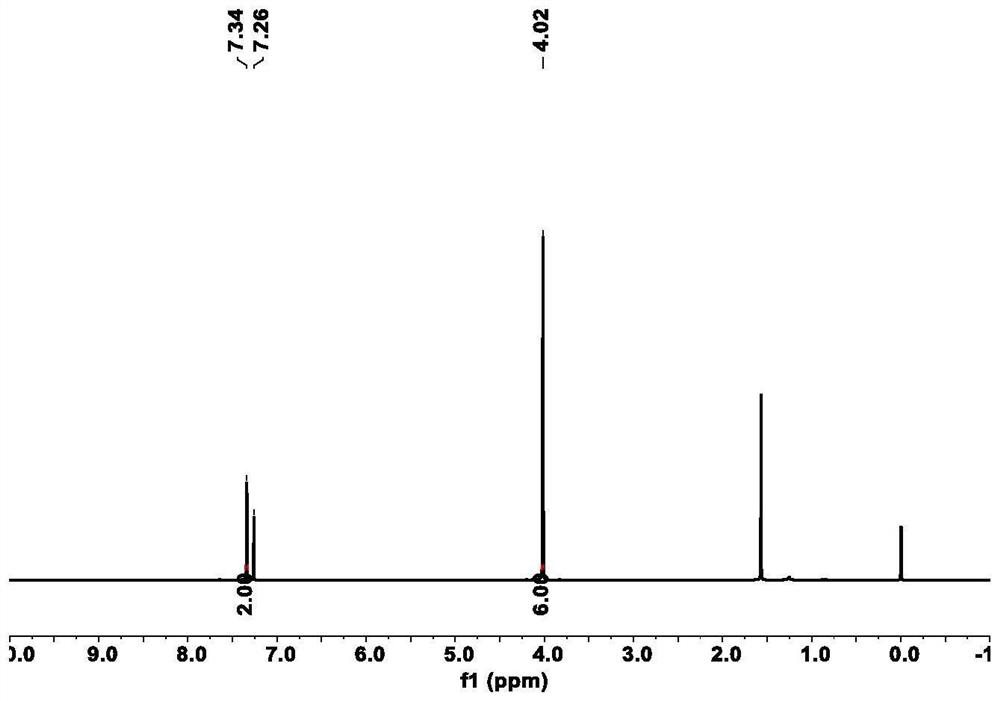
[0066] This example is the preparation of 1,2-dimethoxy-4,5-dinitro
[0067]
[0068] Measure 10mL of 1,2-dimethoxybenzene in the reaction bottle, under the condition of ice bath, slowly add 35mL of fuming nitric acid (95wt%) while stirring, heat the reaction at 85°C for 12h after the reaction rises to room temperature, cool After reaching room temperature, deionized water was added to precipitate a precipitate, which was vacuum filtered, the filter residue was washed with water, and recrystallized from ethanol to obtain yellow needle-like crystals, which were dried in vacuum.
[0069] 1,2-dimethoxy-4,5-dinitro proton NMR spectrum results are as follows figure 1 shown.
Embodiment 2
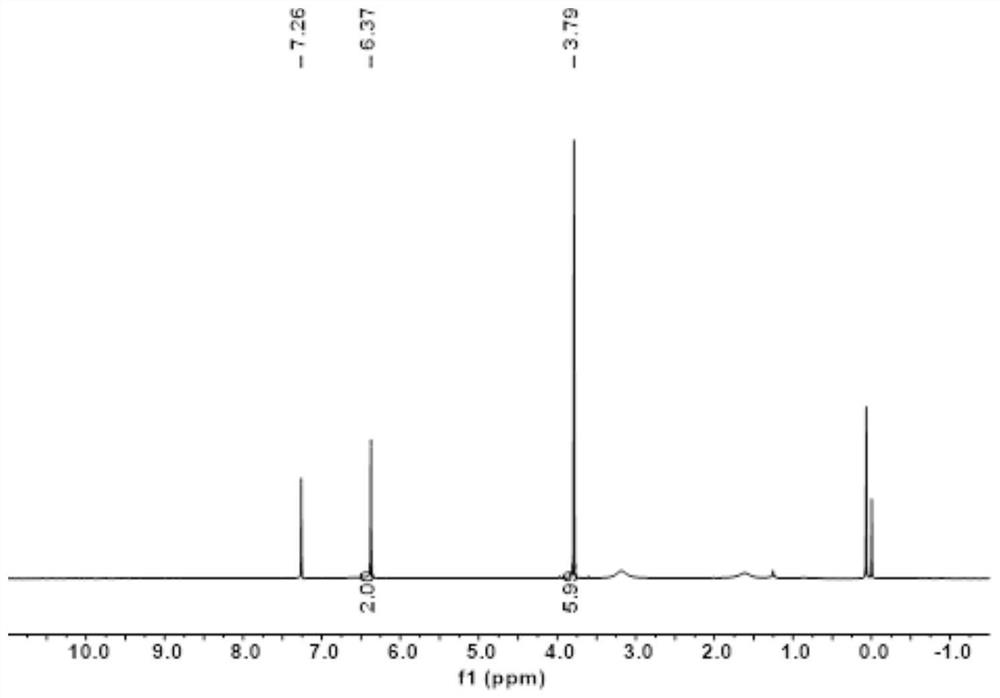
[0071] This example is the preparation of 1,2-dimethoxy-4,5-diaminobenzene
[0072]
[0073] Under the protection of nitrogen, add 1,2-dimethoxy-4,5-dinitrobenzene (1g, 4.4mmol), palladium carbon (150mg, wherein the mass content of palladium is 10%) into the reaction eggplant bottle, Add deoxygenated absolute ethanol (80mL), heat the reaction at 85°C, and add hydrazine hydrate (20mL, 50wt%) under nitrogen protection, react for 16h, vacuum filter after cooling, and filter the filtrate with anhydrous Na 2 SO 4 Drying; distillation under reduced pressure to obtain a light yellow solid product. Yield: 670 mg (3.98 mmol, 90.4%).
[0074] 1,2-dimethoxy-4,5-diaminobenzene H NMR spectrum results are as follows figure 2 shown.
Embodiment 3
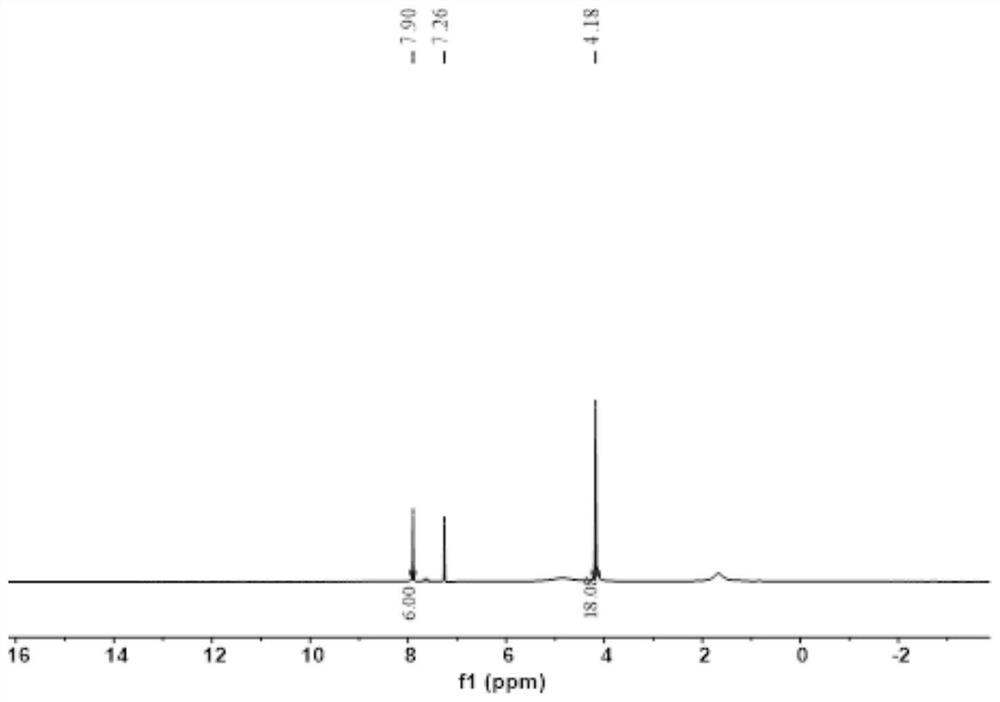
[0076] This example is the preparation of 2,3,8,9,14,15-hexamethoxyquinoxalino[2,3,-a:2',3'-c]phenazine
[0077]
[0078] Under the protection of nitrogen, 1,2-dimethoxy-4,5-diaminobenzene (2.2g, 12.8mmol), cyclohexanone octahydrate (1g, 3.2mmol) were added to the reaction eggplant bottle, Add deoxygenated glacial acetic acid (50mL), heat the reaction at 110°C, reflux for 12h, cool to room temperature, vacuum filter, wash with deionized water (50mL*3), anhydrous methanol (50mL*3) , dried in vacuo to obtain a brown solid product. Yield: 86%.
[0079] Such as image 3 As shown, 2,3,8,9,14,15-hexamethoxyquinoxalino[2,3,-a:2',3'-c]phenazine H NMR spectrum data are: 1 H NMR (400MHz, CDCl 3 ): δ = 4.18 (s, 18H), 7.90 (s, 6H) ppm.
[0080] Such as Figure 4 As shown, 2,3,8,9,14,15-hexamethoxyquinoxalino[2,3,-a:2',3'-c]phenazine carbon NMR spectrum data are: 13 C NMR (100MHz, CDCl 3 ): δ = 56.8, 107.3, 141.2, 141.40, 154.78 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com