Application of atractylenolide III in preparation of medicine for treating non-alcoholic fatty liver disease
A fatty liver disease, non-alcoholic technology, applied in the application field of Atractylodes Lactone III in the preparation of drugs for the treatment of non-alcoholic fatty liver disease, can solve the problem of increasing the risk of coronary artery disease, has not been reported, and the long-term efficacy of pioglitazone needs to be evaluated To achieve the effect of reducing body weight and liver lipid deposition, reducing liver index, and inhibiting lipid deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Animal Experiment of Atractyloderm III in Treating Nonalcoholic Fatty Liver Disease
[0035] 1 Experimental materials
[0036]1.1 Experimental animals and cells
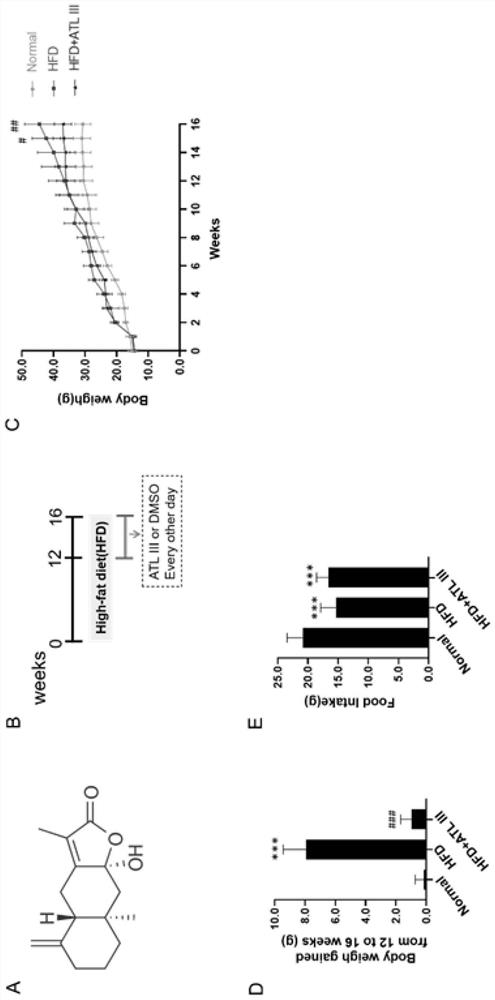
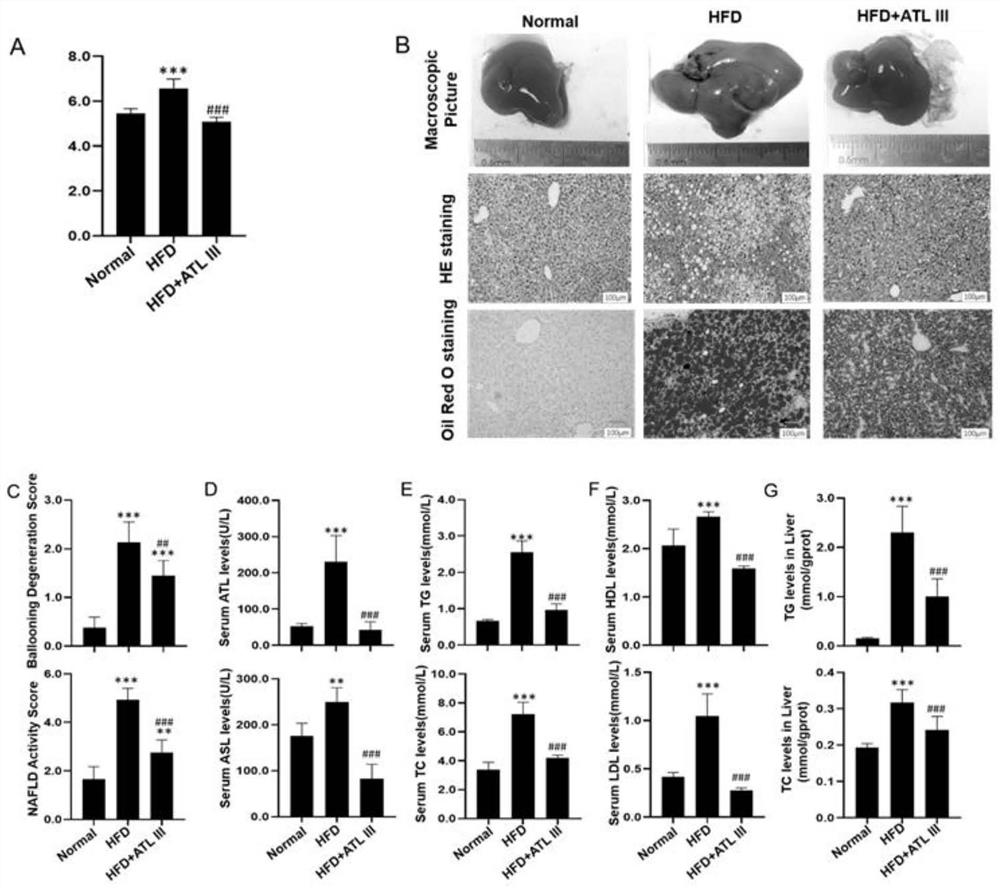
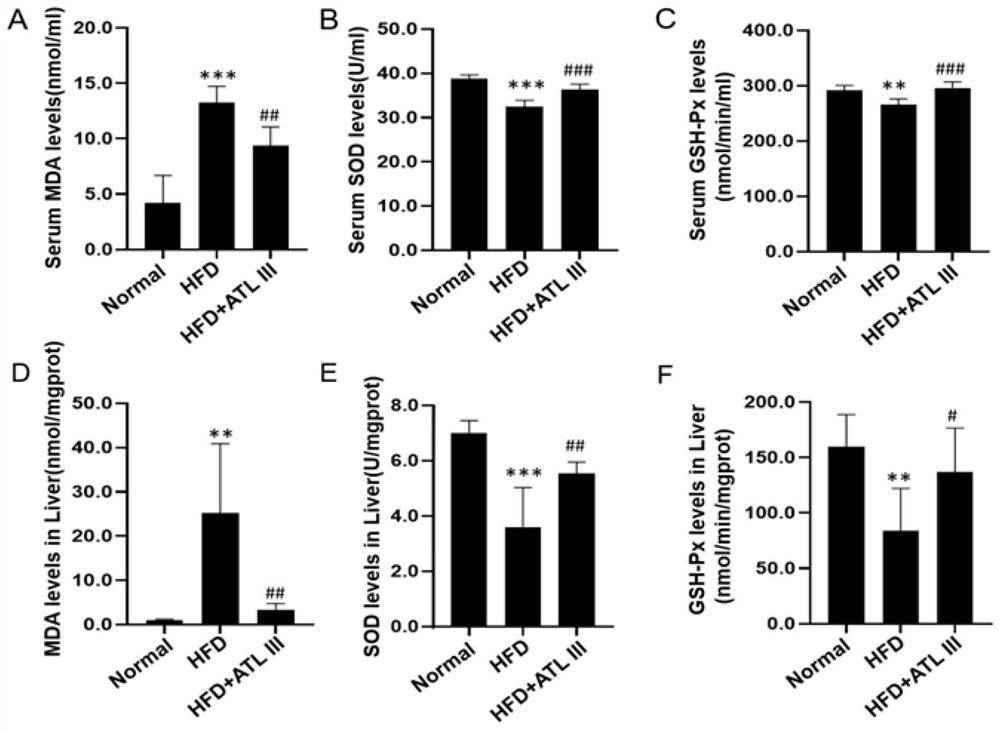
[0037] Thirty 5-week-old male C57BL / 6J mice, weighing 14-18g, were sourced from Shanghai Bikai Experimental Animal Co., Ltd., and the license number for experimental animals is: SYXK (Shanghai) 2020-0009. The mice were raised in an SPF-grade barrier system in the Experimental Animal Center of Shanghai University of Traditional Chinese Medicine at a temperature of (24±2)°C and a humidity of 55%. The mice had free access to feed and drinking water. The animal ethics IACUC number is PZSHUTCM210320005.
[0038] 1.2 Experimental drugs and main reagents
[0039] Atractylodes lactone Ⅲ (ATL Ⅲ) was purchased from Shanghai Tongtian Biotechnology Co., Ltd., 60kcal% fat calorie high-fat mouse food (HF60) was purchased from Daitz Biotechnology (Wuxi) Co., Ltd., fatty acid-free bovine serum albumin (BSA), Ole...
Embodiment 2
[0116] Example 2 Cell Experiment of Atractylolide III Therapeutic Effect
[0117] 1 Experimental materials
[0118] 1.1 Experimental cells
[0119] Human liver cancer cell line HepG2 cells were purchased from Nanjing Kebai Biotechnology Co., Ltd.
[0120] 1.2 Experimental drugs and main reagents
[0121] Atractylodes lactone III (ATL III) was purchased from Shanghai Tongtian Biotechnology Co., Ltd. Fatty acid-free bovine serum albumin (BSA), oleic acid (OA) and palmitic acid (PA) were purchased from Sigma-Aldrich, dimethyl sulfoxide ( DMSO) was purchased from Tauto-Biotech, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin and streptomycin were purchased from Gibco, CCK-8 kit, Compound C, EX 527 were purchased from AbMole, ALT / AST / TG / TC / HDL / LDL / MDA / GSH-Px / SOD / ROS test box, oil red O dye kit, HE dye were purchased from Nanjing Jiancheng Biotechnology Research Institute, RIPA lysate, BCA protein quantification kit , SDS-PAGE gel preparation kit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com