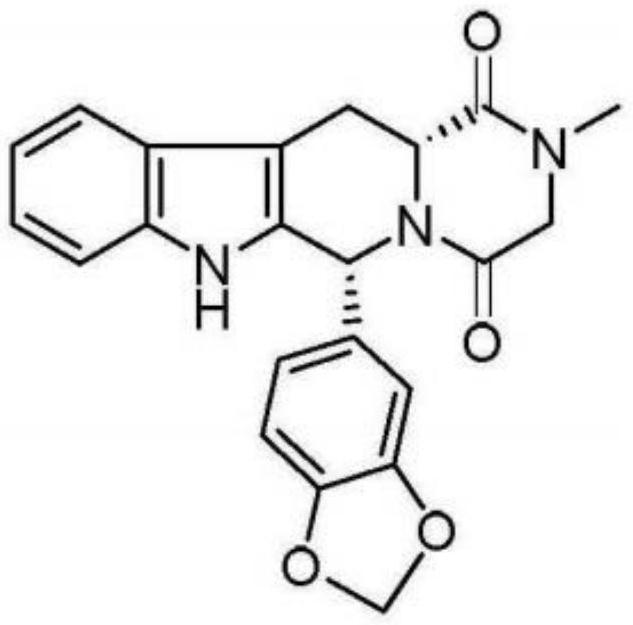
Preparation method of phosphodiesterase inhibitor
A technology for phosphodiesterase and inhibitors, which is applied in the field of preparation of phosphodiesterase inhibitors, can solve the problems that are not conducive to large-scale production and the total yield of products is not high, achieve the optimization of reaction conditions and operation methods, and ensure product yield. efficiency and purity, and the effect of shortening the reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
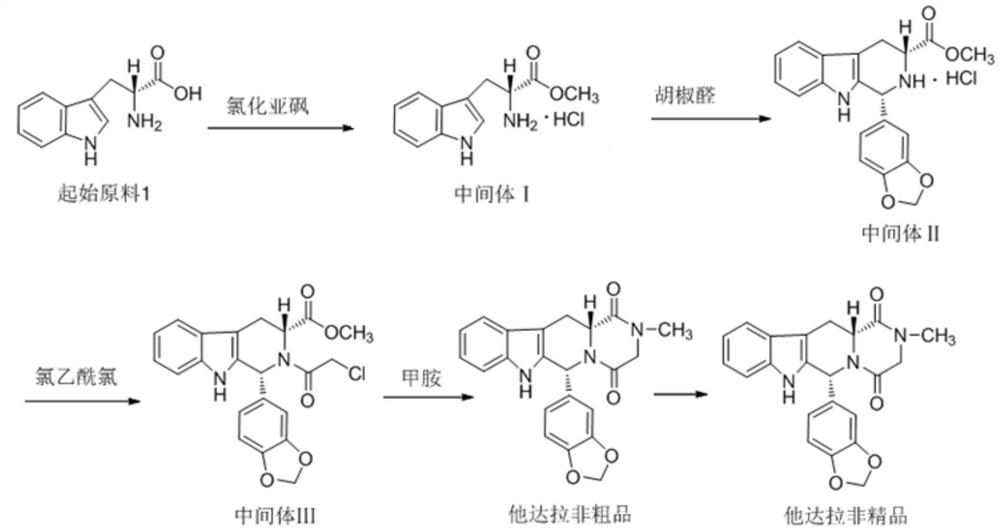
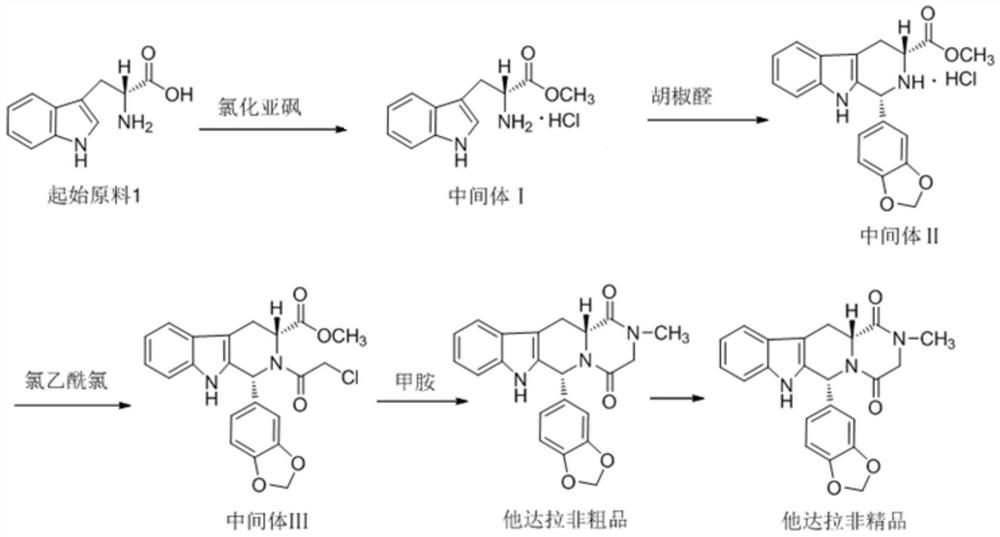
[0027] Preparation of Intermediate II
[0028] Add 1.8L of methanol to the reaction kettle, add 361g of starting material 1, add 2.65mol of thionyl chloride dropwise at 20±5°C, after the drop is complete, heat to reflux at 65±3°C for 1 hour, and monitor the start After the reaction of raw material 1 was completed, the solvent was evaporated under reduced pressure, then 1.8L of isopropanol and 265g of piperonal were added, stirred and heated to reflux for 7h, the mixture was cooled to 10±5°C, stirred and crystallized for 2h, centrifuged, and vacuum-dried to obtain The solid powder intermediate II was 596g, the yield was 87.2%, the purity was 99.10%, and the isomer impurity was not detected.
Embodiment 2
[0030] Preparation of Intermediate II
[0031] Add 1.8L of methanol into the reaction kettle, add 353g of starting material 1, add 1.73mol of thionyl chloride dropwise at 20±5°C, after the drop is complete, heat to reflux at 65±3°C for 1 hour, and monitor the start After the reaction of raw material 1 was completed, the solvent was evaporated under reduced pressure, then 1.8L of isopropanol and 260g of piperonal were added, stirred and heated to reflux for 8 hours, the mixture was cooled to 10±5°C, stirred and crystallized for 2 hours, centrifuged, and vacuum-dried to obtain The solid powder intermediate II was 554g, the yield was 82.8%, the purity was 98.53%, and the isomer impurity was not detected.
Embodiment 3
[0033] Preparation of Intermediate II
[0034] Add 1.8L of methanol into the reaction kettle, add 359g of starting material 1, and add 1.76mol of thionyl chloride dropwise under the condition of 20±5°C. After dropping, heat to reflux at 65±3°C for 2 hours, monitor After the reaction of the starting material 1 was completed, the solvent was evaporated under reduced pressure, then 1.8L of isopropanol and 317g of piperonal were added, stirred and heated to reflux for 10h, the mixture was cooled to 10±5°C, stirred and crystallized for 2h, centrifuged, and vacuum-dried , 569g of solid powder intermediate II was obtained, the yield was 83.6%, the purity was 99.22%, and the isomer impurity was not detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com