Seven RNA virus nucleic acid detection quality control products and preparation method thereof
A technology of RNA virus and quality control products, which is applied in the fields of clinical laboratory science and applied molecular biology, can solve the problems of reduced yield of virus-like particles, difficulty in sharing quality control products, and limited application range, etc., to overcome low preparation efficiency, High safety, simple and efficient preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
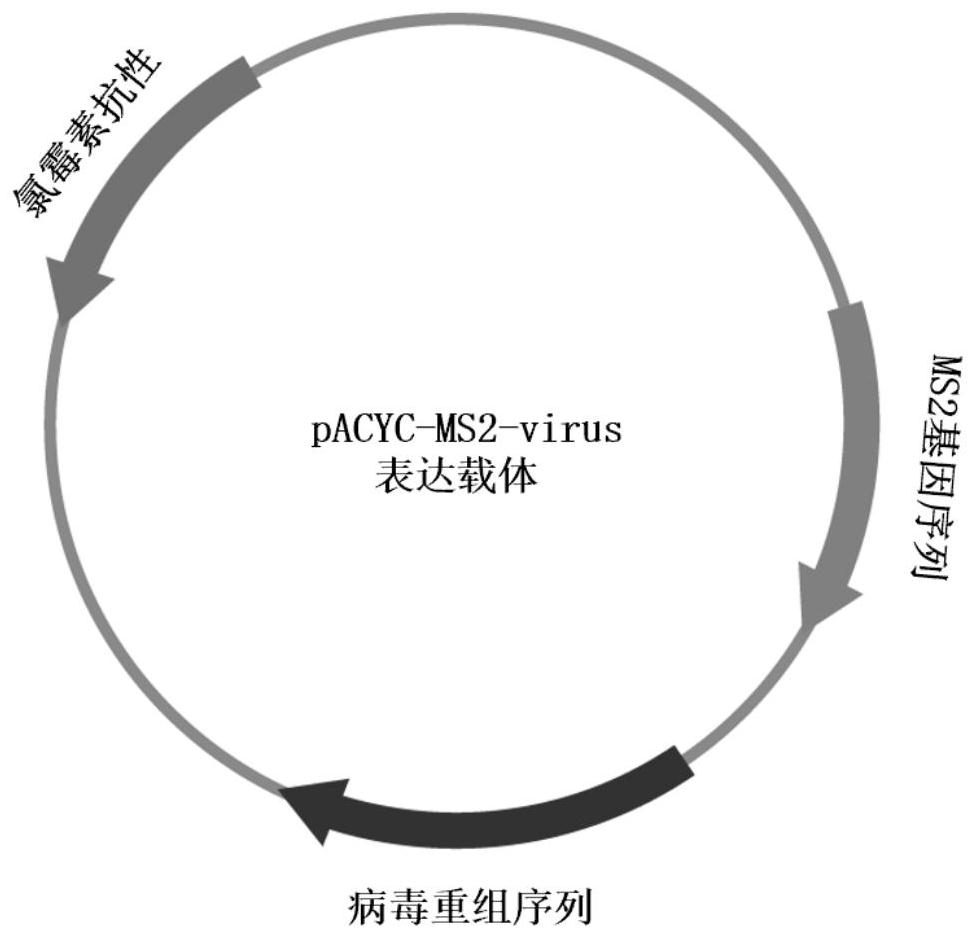
[0103] Example 1 Construction of pACYC-MS2-virus vector construction
[0104] 1. Construction of pACYC-MS2 recombinant plasmid
[0105] 1) Obtain the gene sequence encoding MS2 phage capsid protein and mature enzyme protein from the GenBank database, and design and add BamHI and NotI restriction enzyme site sequences at both ends of the sequence. The sequence information is shown in SEQ ID No.1; The design sequence was artificially synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. and inserted into the PUC-SP vector.
[0106] 2) Use the restriction endonucleases BamHI and NotI to double-digest the pUC-57 and pACYCDuet-1 plasmids respectively, and the restriction enzyme digestion system is as follows:
[0107] Table 2
[0108]
[0109]
[0110] Digest at 37°C for 1 hour, inactivate restriction enzymes at 80°C for 5 minutes, perform 1% agarose gel electrophoresis on the digested target products, and recover and purify by cutting the gel. The products are named MS...
Embodiment 2
[0126] Embodiment 2 Preparation of MS2 virus-like particles containing seven kinds of RNA virus nucleic acids
[0127] 1) The pACYC-MS2-virus recombinant plasmids of the seven viruses successfully sequenced were transformed into expressive BL21(DE3) E. Coli competent cells.
[0128] 2) Positive clones were inoculated into 5 mL of LB liquid medium containing chloramphenicol (final concentration 34 μg / mL), cultured for about 4-6 hours, and 2 mL of bacterial liquid was added to 200 mL of LB liquid medium for expansion, 37 ° C, 250 rpm Shake culture for about 2-3 hours, when the OD 600 value of the bacterial solution is about 0.6-0.8 with a spectrophotometer, add isopropylthio-β-D-galactoside with a final concentration of 1mmol / L to the bacterial solution (IPTG), 37° C., 250 rpm and continue shaking culture for about 12-16 hours to induce the expression of MS2 virus-like particles.
[0129] 3) Centrifuge at 9500rpm for 3min at 4°C, discard the supernatant, retain the bacterial pe...
Embodiment 3
[0134] Embodiment 3 MS2 virus-like particle verification and quantification
[0135] 1. DNase Ⅰ and RNase A enzyme digestion verification
[0136] The MS2 virus-like particle solution obtained in Example 2 was digested with DNase I and RNase A with final concentrations of 200 U / mL and 500 U / mL respectively, and the sample loading system was as follows:
[0137] Table 6
[0138]
[0139] Digest for 2 hours in a water bath at 37°C to remove residual nucleic acid, and perform 1% agarose gel electrophoresis analysis.
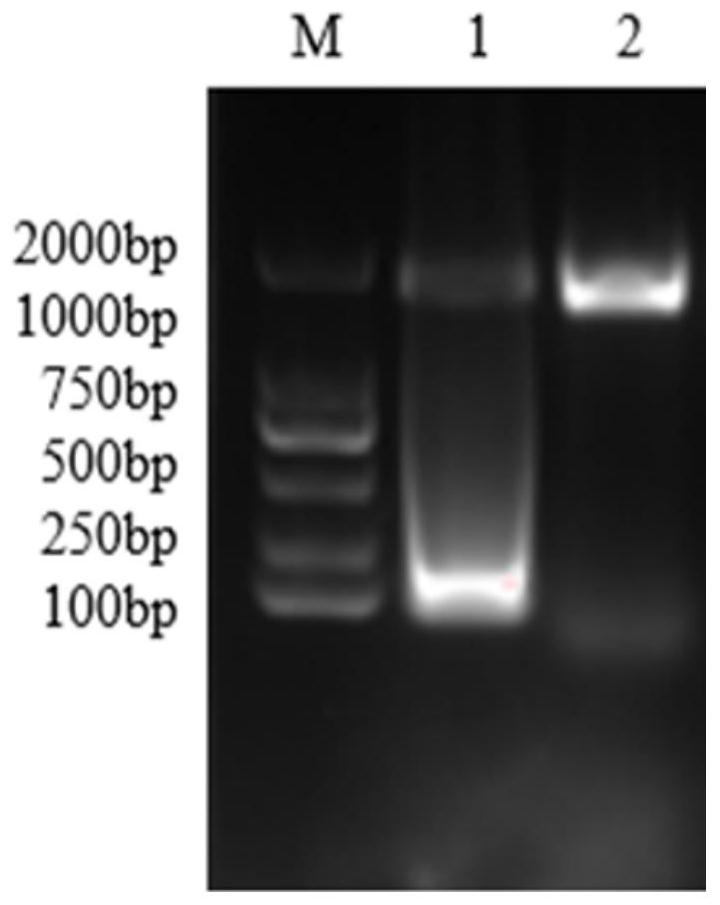
[0140] Taking the EV71 virus as an example, through electrophoresis analysis (as attached to the instruction manual) image 3 As shown), it can be seen that the EV71MS2 virus-like particles purified in Example 2 are a bright single band after being digested by DNase I and RNase A, while the virus-like particles that have not been digested by DNase I and RNase A are due to the residual nucleic acid adhesion on the surface. The "tailing phenomenon" appears, indi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com