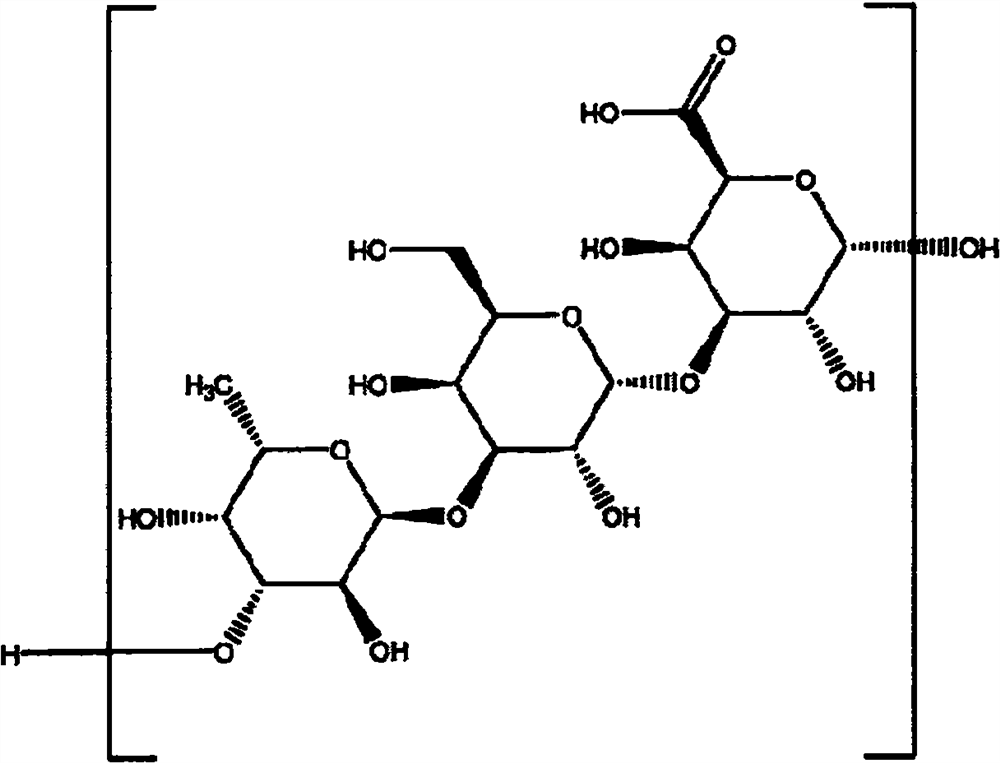
Application of natural polymeric polysaccharide in preparation of sample treatment solution and application of natural polymeric polysaccharide in immunochromatography detection kit
A detection kit and sample processing solution technology, applied in the field of medical testing, can solve problems in lateral flow detection, formation of rejection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of sample treatment solutions containing different concentrations of natural polymeric polysaccharide Biosaccharide Gum-1
[0035]In the borate buffer solution with pH 8.0-8.5, add Tween 20 at a final concentration of 1% (v / v), 0.9% sodium chloride at a final concentration and proclin300 at a final concentration of 0.03%, add Biosaccharide Gum-1 , prepare the treatment solution comprising Biosaccharide Gum-1 of different working concentrations, as follows:
[0036] Treatment solution 1: boric acid buffer solution with a pH of 8.0-8.5, Tween 20 of 1% (v / v), final concentration of 0.9% sodium chloride and proclin300 of 0.03% final concentration, Biosaccharide Gum-1 concentration of 0.2 %(v / v).
[0037] Treatment solution 2: boric acid buffer solution with a pH of 8.0-8.5, Tween 20 of 1% (v / v), final concentration of 0.9% sodium chloride and proclin300 of 0.03% final concentration, Biosaccharide Gum-1 concentration of 0.4 %(v / v).
[0038] Treatment...
Embodiment 2
[0041] The preparation of embodiment 2 fluorescent immunochromatography test strips
[0042] Spray the new coronavirus antigen RBD protein and the rabbit polyclonal antibody of the new coronavirus antigen RBD protein on the nitrocellulose membrane with a gold spray machine to form parallel detection T lines and quality control C lines, and then dry; Dissolve the antigen-labeled fluorescent microspheres in the spraying buffer at a concentration of 2.3 mg / mL, and uniformly spray on the marking pad at a spraying speed of 4 μL / cm. The spraying buffer contains 0.3-0.7% BSA and 0.2- 0.3% Tween-20 concentration in 0.01-0.03M borate buffer, pH 8.0-8.5.
[0043] For the T line and C line, the spraying speed of the gold dot film machine is 1μL / cm, the T line is the new coronavirus antigen RBD protein with a concentration of 1mg / mL, and the C line is the new coronavirus antigen RBD protein with a concentration of 1mg / mL. Rabbit polyclonal antibody.
[0044] Then connect and assemble th...
Embodiment 3
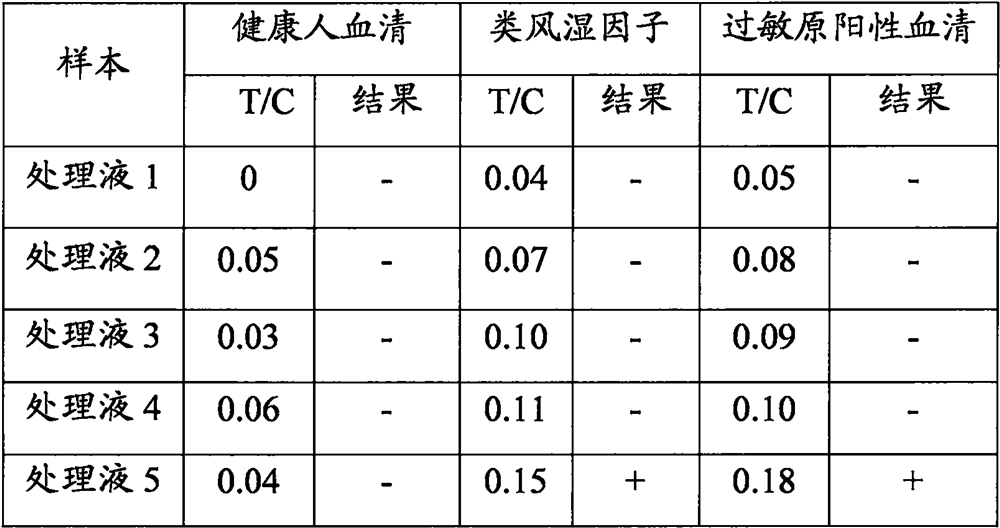
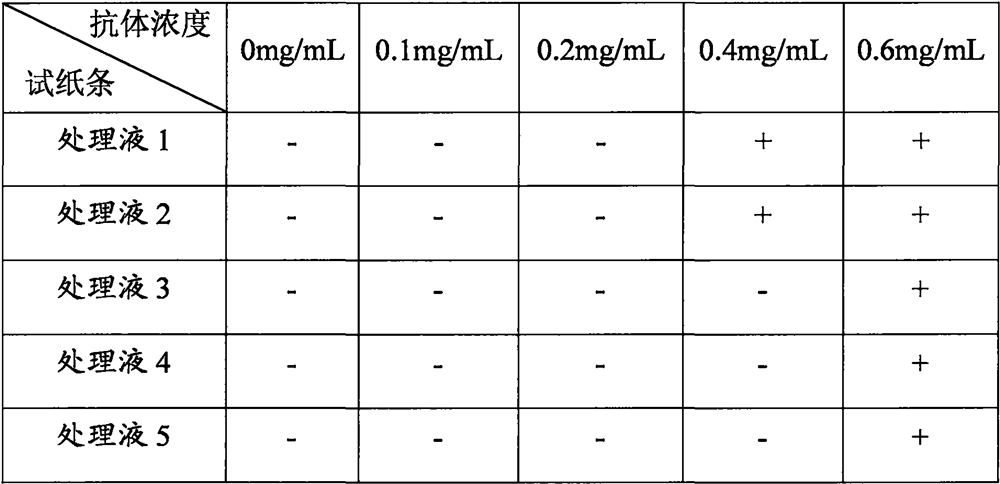
[0045] Specificity determination of embodiment 3 sample treatment liquid
[0046] (1) Sample preparation: 100 μL of human serum was collected.
[0047] (2) Detection: Take the test strip, equilibrate at room temperature for 10 minutes, open the package and take out the test strip; mix 50 μL of serum and 50 μL of the sample treatment solution (various sample treatment solutions in Example 1), and add 90 μL of the mixed solution to the Put it on the sample pad of the new coronavirus protein antibody detection test strip, and after standing for 15 minutes, insert the test strip into the dry fluorescent immunoassay instrument in the direction of the arrow to determine the result. Compare the results of parallel experiments under different sample treatment solution conditions.
[0048] (3) Judgment of the result: when the T / C of the test strip is <0.12, it is judged as negative and recorded as "-"; when the T / C of the test strip is <0.12, it is judged as positive and recorded as "...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com