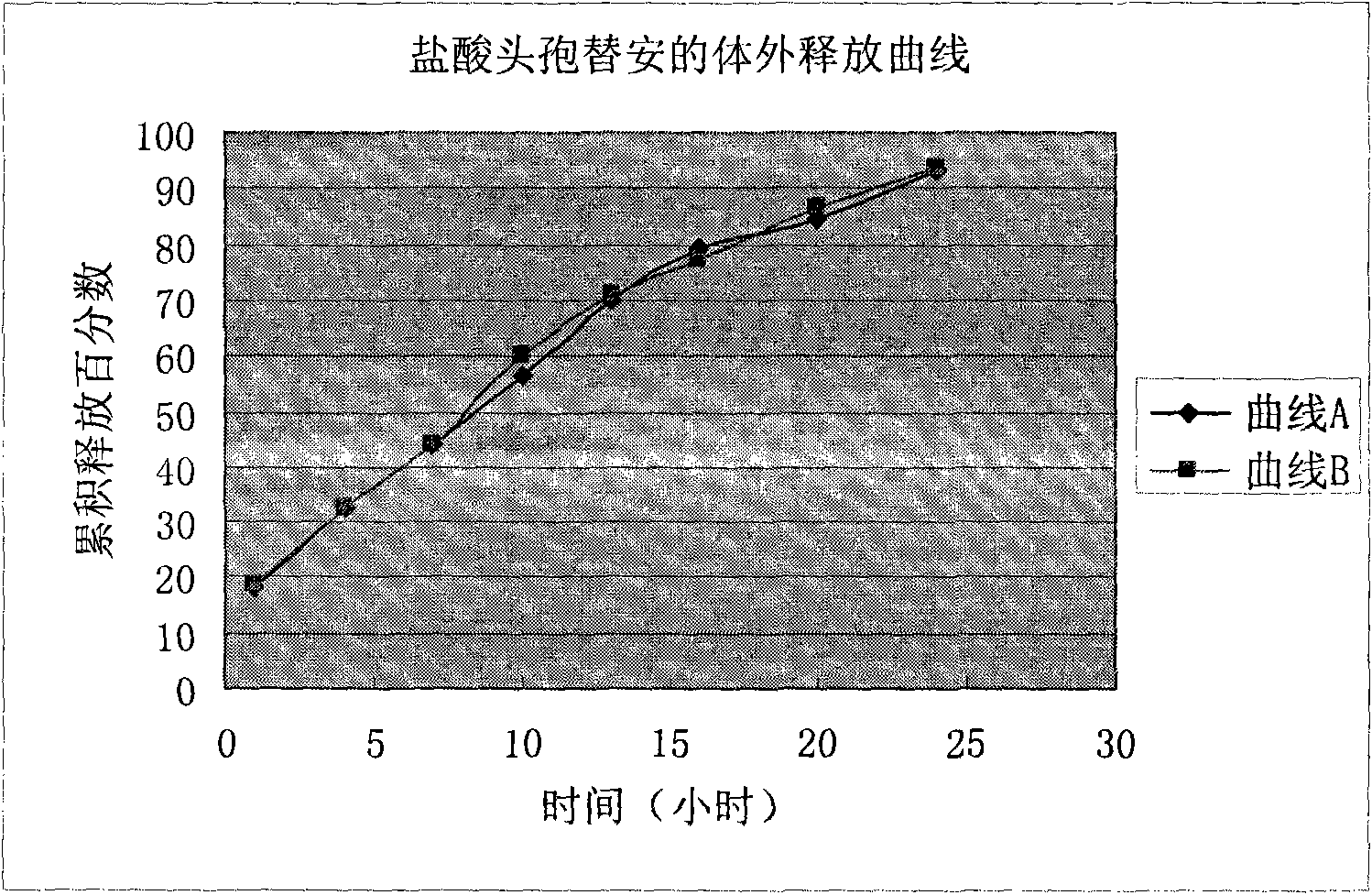
Patents
Literature
40results about How to "Easy to reconstitute" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
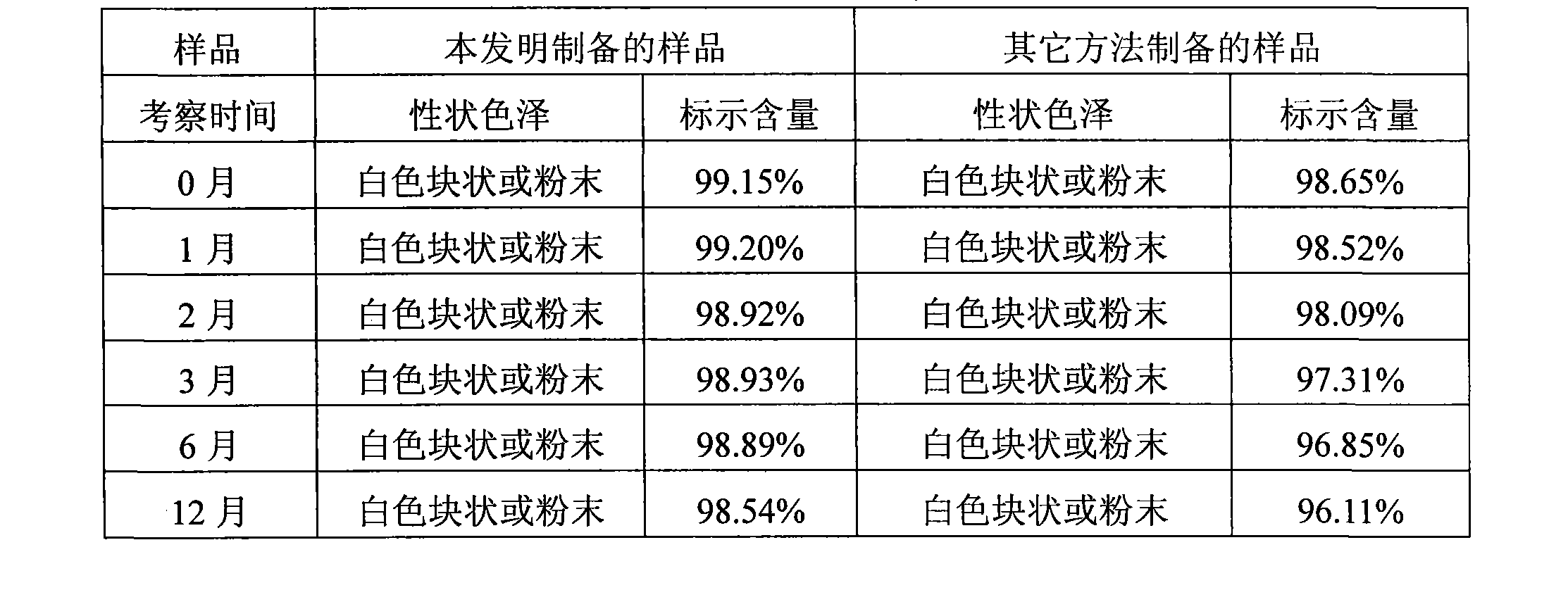
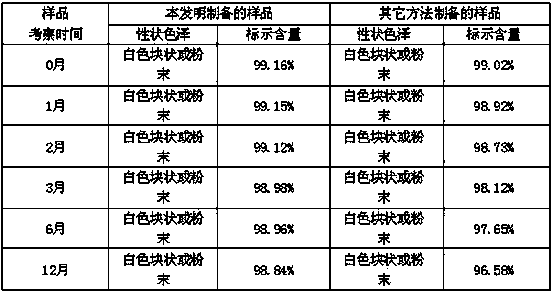
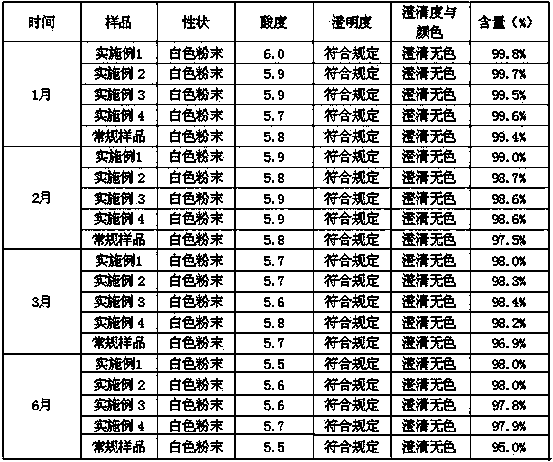
Tropisetron preparation for injection and preparation method thereof
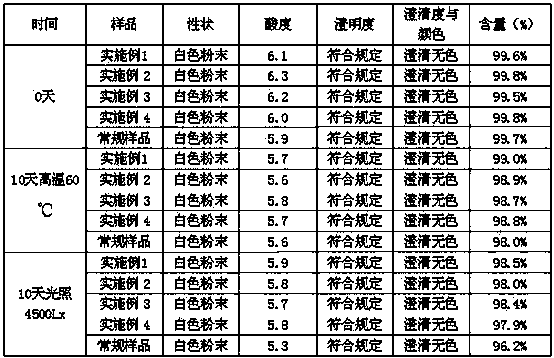
InactiveCN101444508AFlat surfaceNot crackedDigestive systemMacromolecular non-active ingredientsMANNITOL/SORBITOLActive component
The invention provides a tropisetron preparation for injection. The main active components of the tropisetron preparation are tropisetron, beta-cyclodextrin or hydroxypropyl-beta-cyclodextrin and mannitol. The method comprises the following steps: dissolving tropisetron hydrochloride and the beta-cyclodextrin or the hydroxypropyl-beta-cyclodextrin, then adding the mannitol for dissolving, adjusting the pH value with citric acid-disodium hydrogen phosphate buffer solution to obtain a liquid medicine, quickly prefreezing the liquid medicine after filling, and then lyophilizing to obtain the tropisetron preparation. The tropisetron preparation for injection has flat surface, is fine and smooth and uniform, and is free from crack, breakage and sticking to bottles. The tropisetron preparation is a white loose block which is well formed and very easily dissolved, has good redissolution performance, clean and transparent solution, stable product quality, and practicability.
Owner:海南瑞基药物研究有限公司
Methods of enhancing solubility of agents
InactiveUS20080102109A1Readily lyophilizedEasy to diluteBiocideFactor VIISolubilityCombinatorial chemistry
This invention concerns novel methods of enhancing the solubility of a compound. Compositions prepared using such methods are also disclosed. Compositions prepared using the methods have various advantages over conventionally known compositions.
Owner:FORMATECH
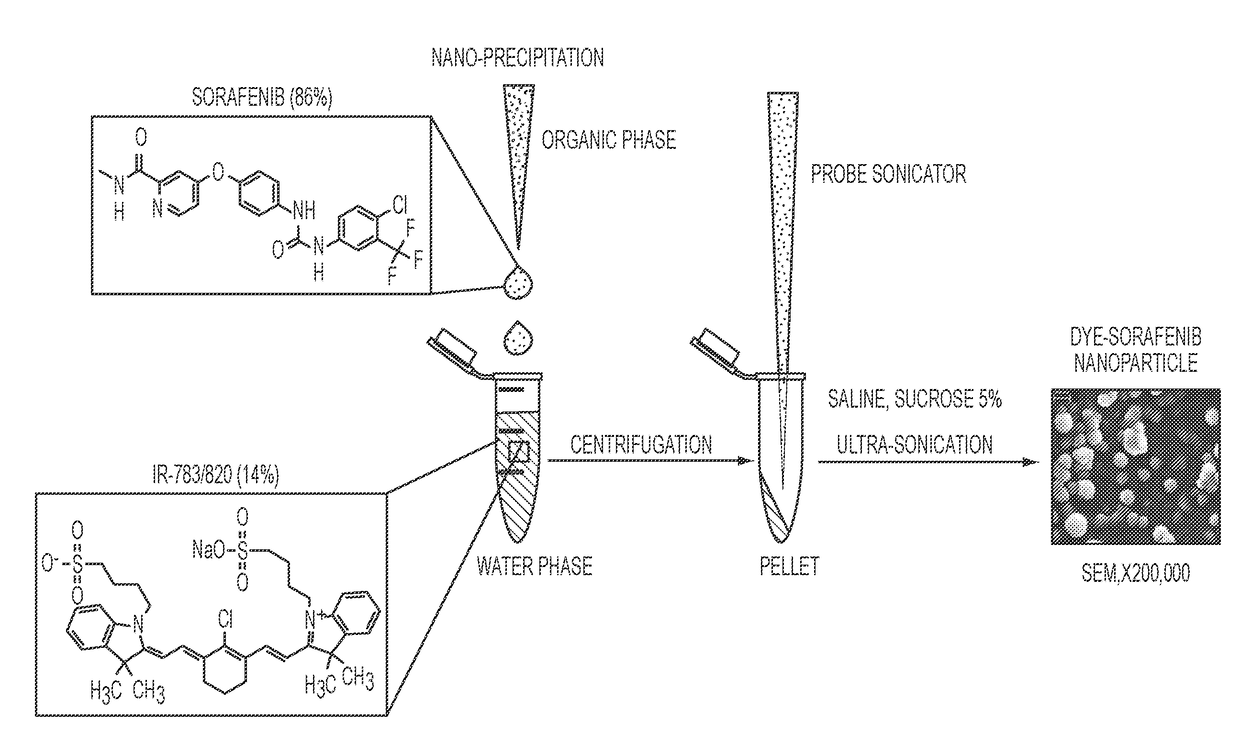
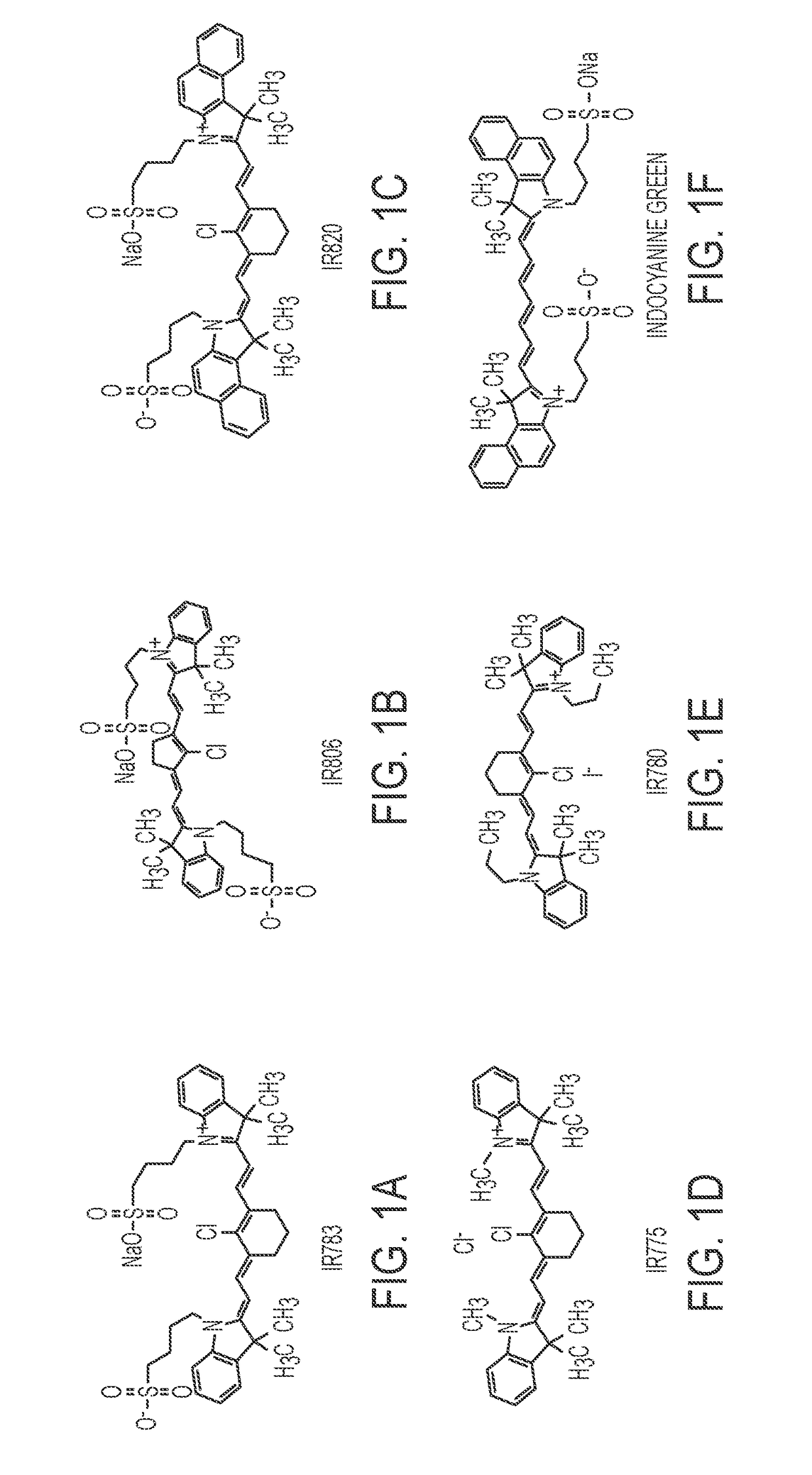
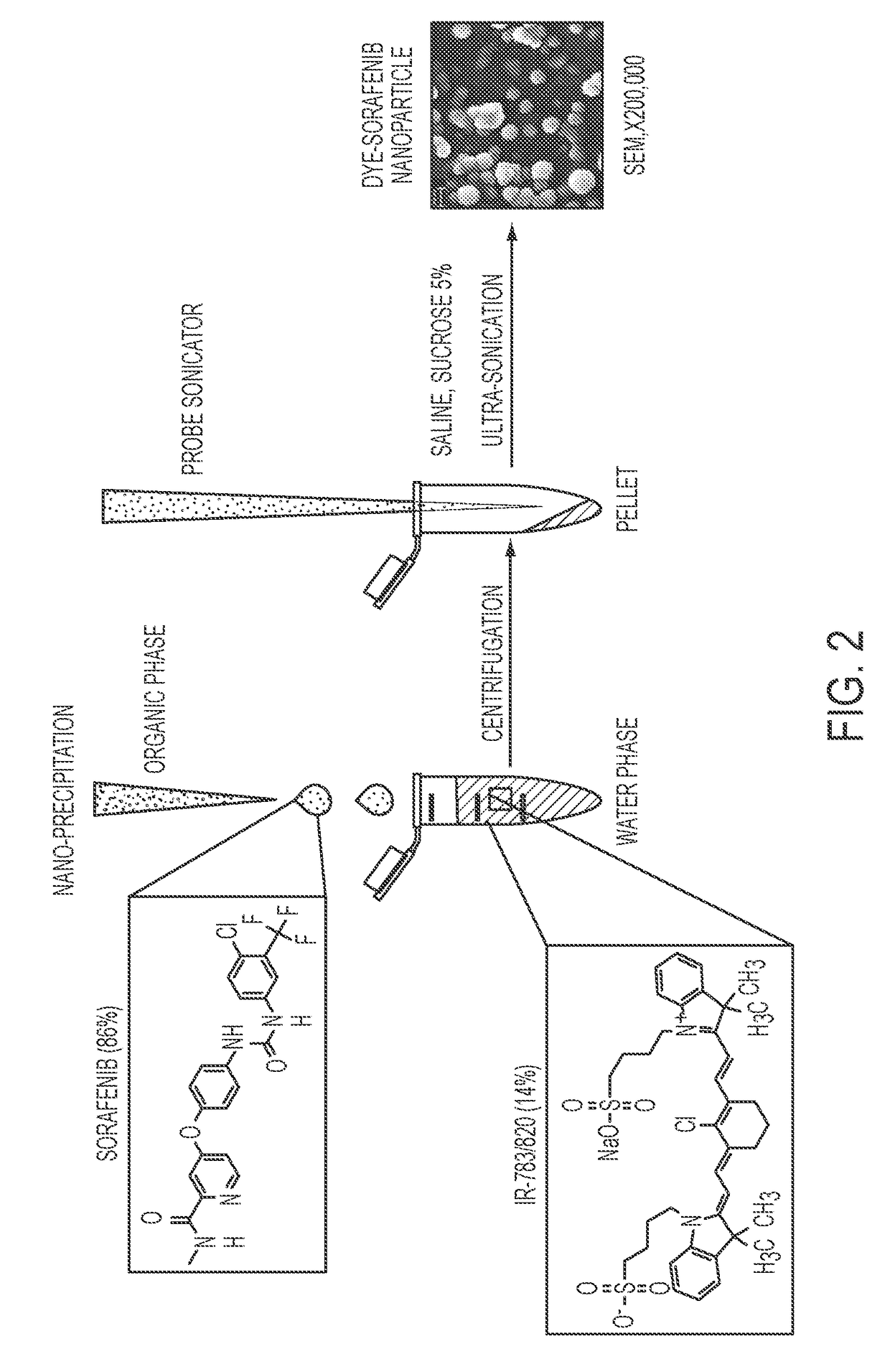
Dye-stabilized nanoparticles and methods of their manufacture and therapeutic use
InactiveUS20180021259A1Increase surface chargeEasily lyophilzedPowder deliveryMolecular designTherapeutic effectIn vivo
Described herein are nanoparticles which are largely made of (e.g., 90 wt. %) hydrophobic drugs and are stabilized by water soluble dyes. The nanoparticles can range in size from 30 nm to 150 nm and have highly negative surface charge (e.g., −55 mV). These nanoparticles are highly soluble in water, stable for days in PBS buffer and can be easily lyophilzed and reconstituted in water. Using quantitative self-assembly prediction calculations, topochemical molecular descriptors were identified and validated as highly predictive indicators of nano-assembly, nanoparticle size, and drug loading. The resulting nanoparticles selectively targeted kinase inhibitors to caveolin-1-expressing human colon cancer and autochthonous liver cancer models to yield striking therapeutic effects while avoiding pERK inhibition in healthy skin. The nanoparticles exhibited remarkable anti-tumor efficacy in vitro and in vivo in models of hepatocellular carcinoma.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
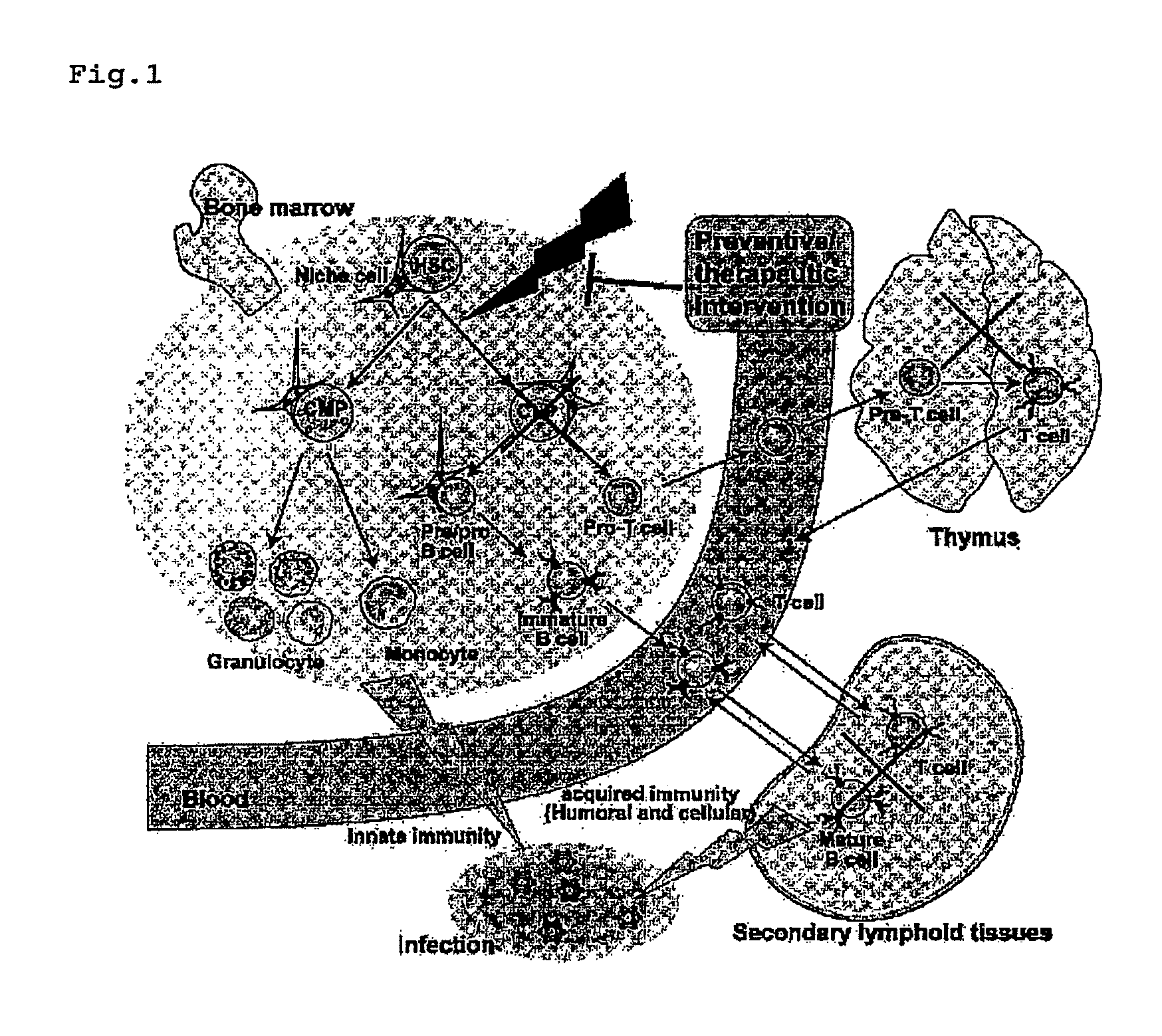
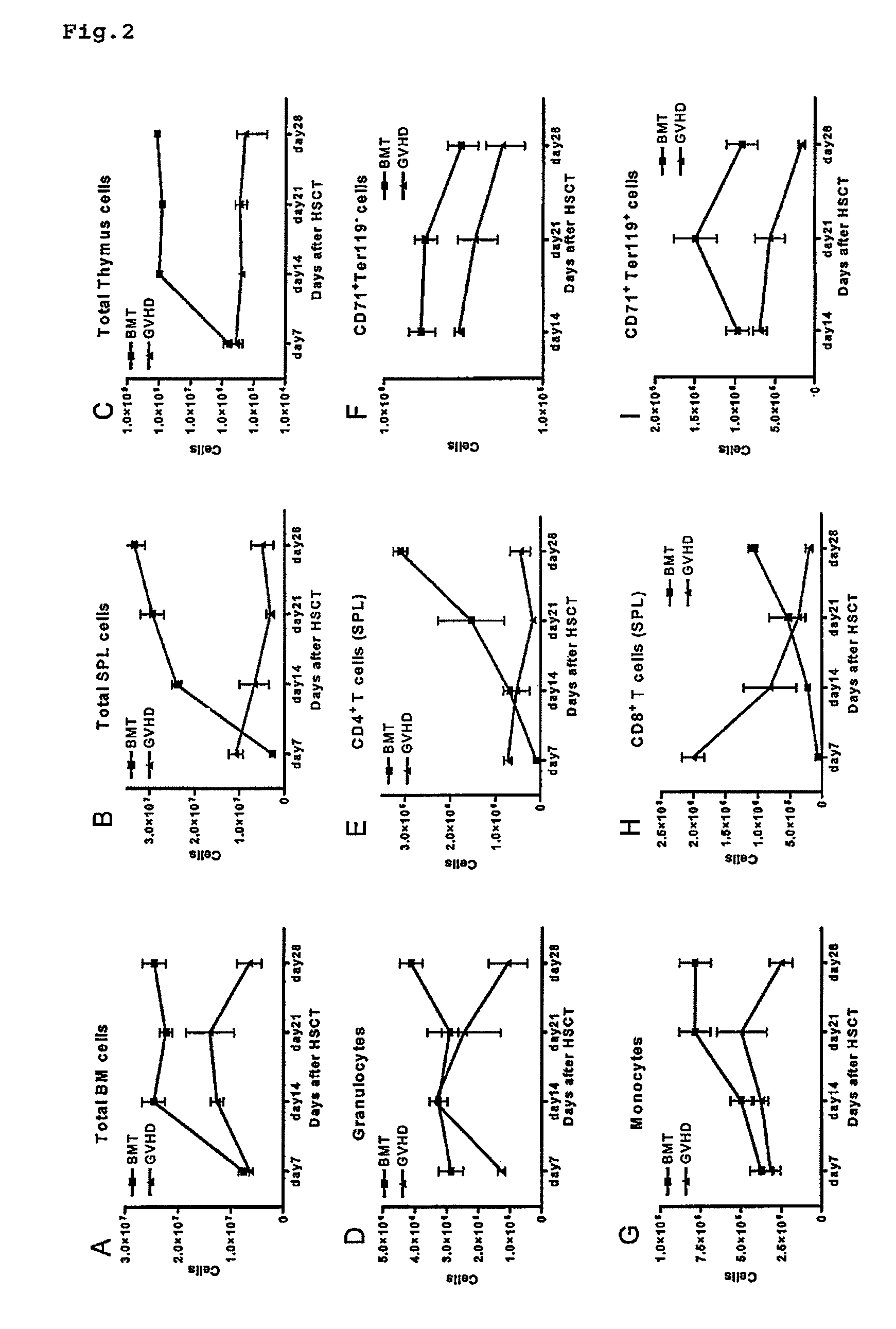
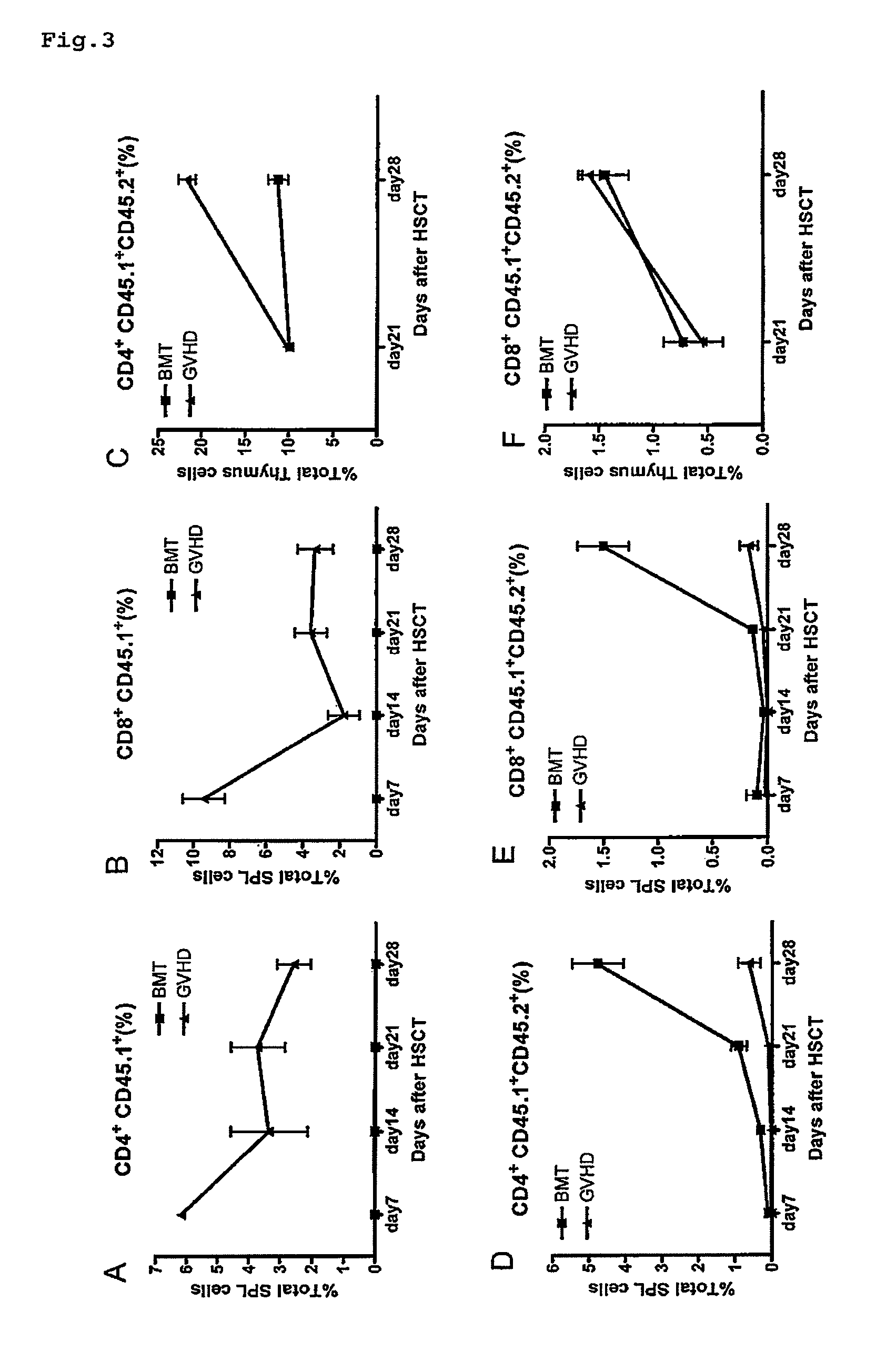
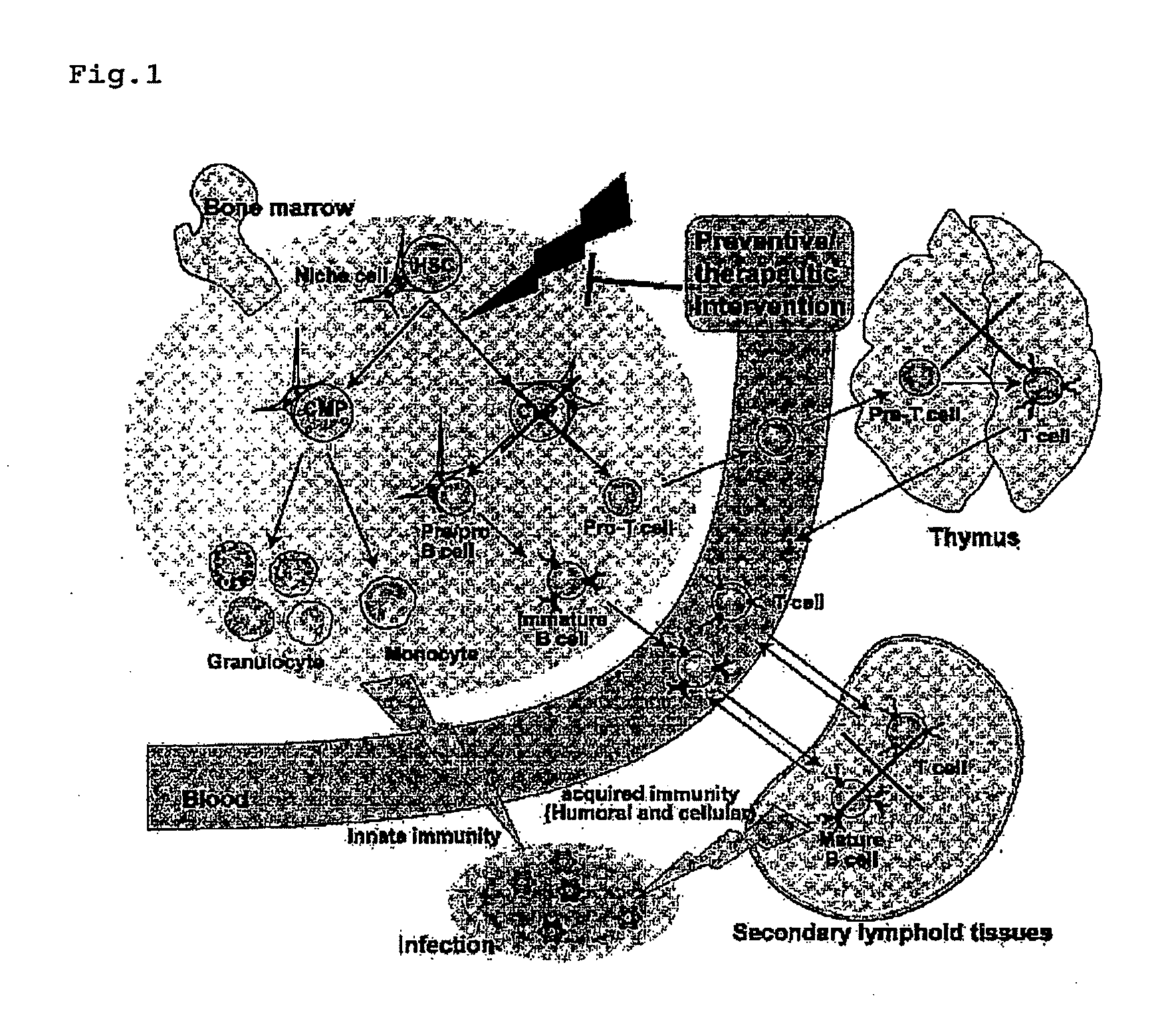
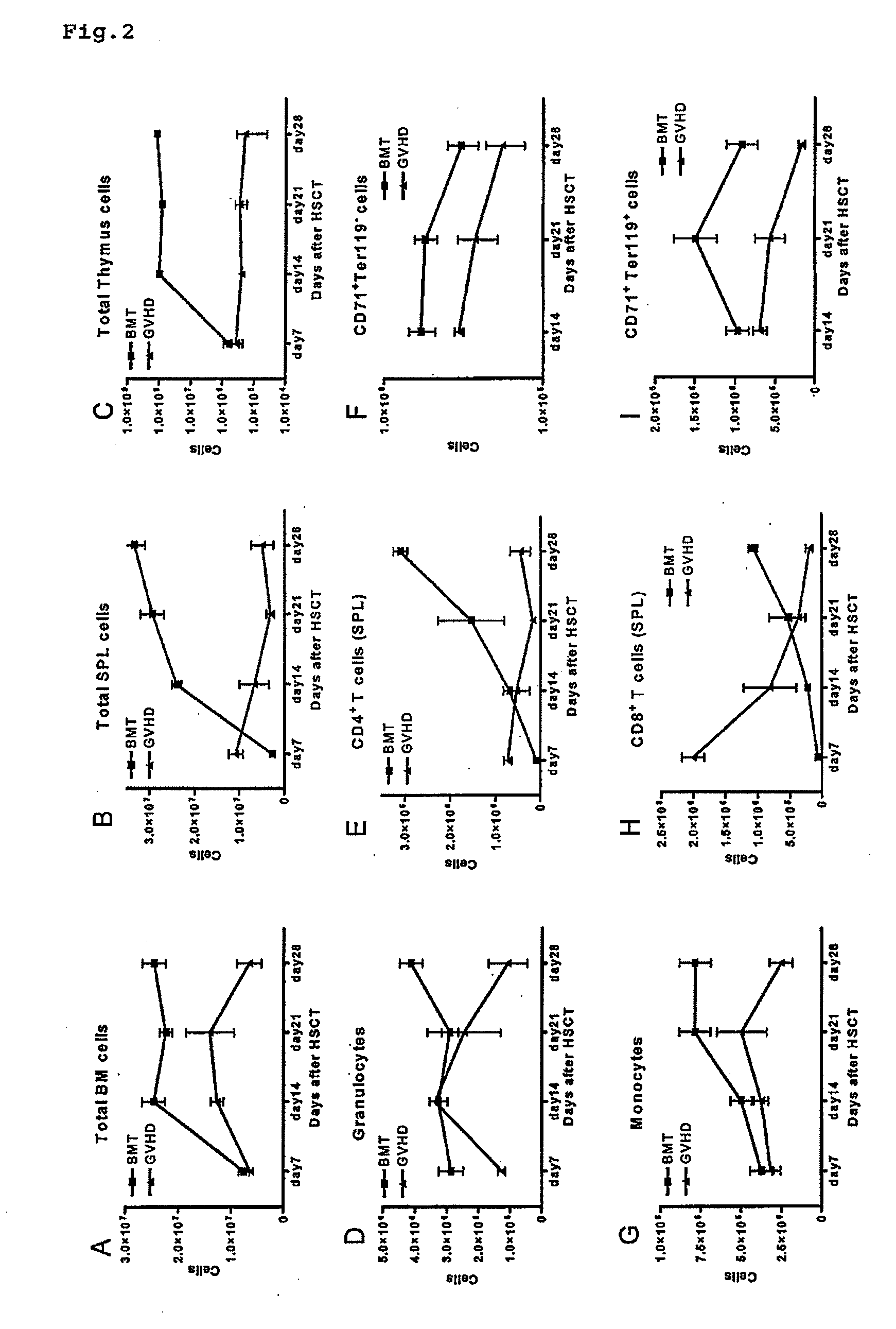
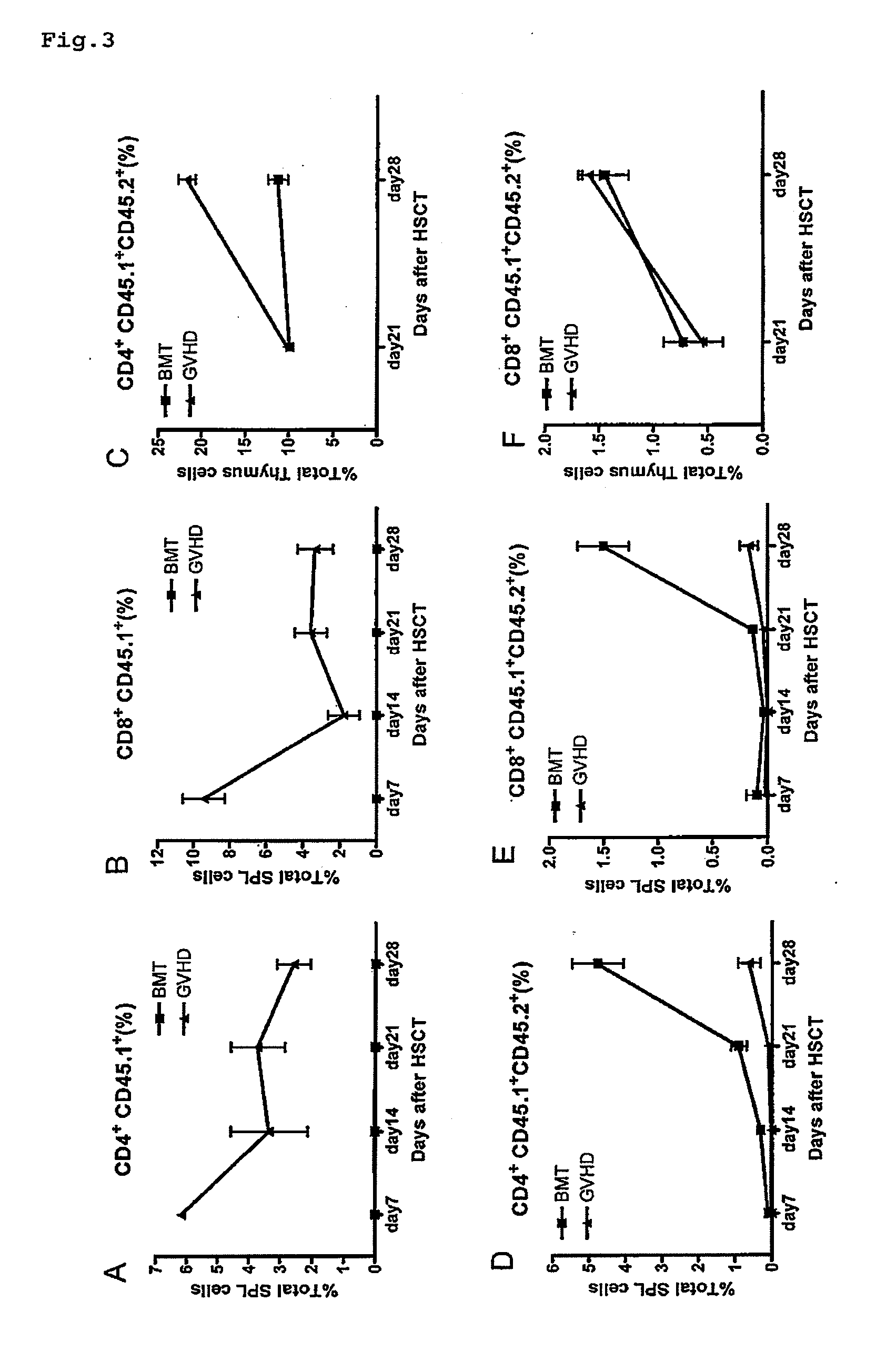
Immunological reconstitution promoter or prophylactic agent for infections each of which maintains graft-versus-tumor effect
The object of the invention is to provide an immunological reconstitution promoter or a prophylactic agent for infections for use in allogeneic hematopoietic stem cell transplantation therapy for tumors. The promoter or prophylactic agent enables the amelioration of delayed immune reconstitution or the prevention of infection following transplantation, while maintaining the GVT effect of allogeneic hematopoietic stem cell transplantation. Specifically, in a transplant patient in whom immune reconstitution is delayed, such reconstitution can be promoted by administering, at an early stage following transplantation, a substance capable of depleting CD4+ cells. Early completion of infection management in the patient and improvement in the survival rate are anticipated as a result. In addition, the risk of complications associated with allogeneic hematopoietic stem cell transplantation is reduced, enabling more widespread use of this therapy.
Owner:KOUJI MATSUSHIMA
Officinal composition of vidarabine monophosphate for injection
ActiveCN103599080AEasy to controlSpray bottlePowder deliveryOrganic active ingredientsActive componentVidarabine Monophosphate
The invention relates to an officinal composition of vidarabine monophosphate for injection, wherein the main active components of the composition comprise vidarabine monophosphate and lysine.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Palonosetron preparation for injection and preparation method thereof
InactiveCN103845295AFlat surfaceNot crackedPowder deliveryOrganic active ingredientsMedicineFreeze-drying
The invention belongs to the technical field of medicines, and particularly relates to palonosetron hydrochloride freeze-dried powder injection. The invention also relates to preparation of the freeze-dried powder injection. By adding beta-cyclodextrin or hydroxypropyl beta-cyclodextrin and mannitol, the palonosetron preparation has the characteristics of being smooth in surface, delicate, uniform, free of cracks, breakage and adhesion to a bottle, white in color, shaped like a loose piece, good in formation, also good in dissolubility, quite easy to dissolve, clear and transparent in solution and stable in product quality.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Immunological reconstitution promoter or prophylactic agent for infections each of which maintains graft-versus-tumor effect
ActiveUS20120027748A1Good effectPromote infectionAntibacterial agentsAntimycoticsCvd riskWilms' tumor
The object of the invention is to provide an immunological reconstitution promoter or a prophylactic agent for infections for use in allogeneic hematopoietic stem cell transplantation therapy for tumors. The promoter or prophylactic agent enables the amelioration of delayed immune reconstitution or the prevention of infection following transplantation, while maintaining the GVT effect of allogeneic hematopoietic stem cell transplantation. Specifically, in a transplant patient in whom immune reconstitution is delayed, such reconstitution can be promoted by administering, at an early stage following transplantation, a substance capable of depleting CD4+ cells. Early completion of infection management in the patient and improvement in the survival rate are anticipated as a result. In addition, the risk of complications associated with allogeneic hematopoietic stem cell transplantation is reduced, enabling more widespread use of this therapy.
Owner:KOUJI MATSUSHIMA
Liver fibrosis marker control material and preparation method thereof
InactiveCN109443876AImprove uniformityImprove stabilityChemiluminescene/bioluminescencePreparing sample for investigationMedicineDiluent
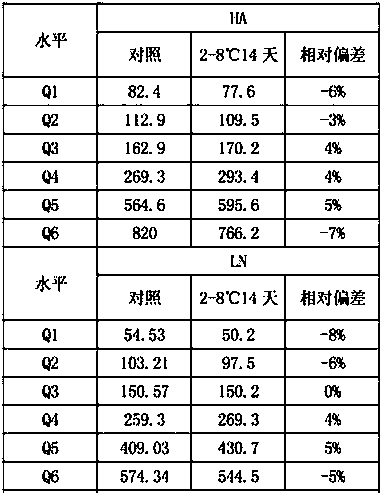
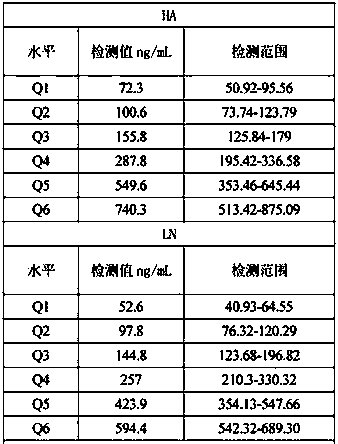
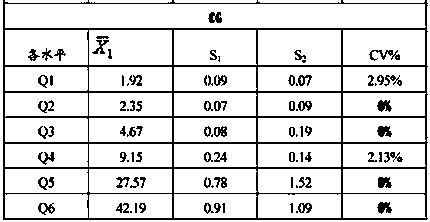
The invention discloses a liver fibrosis marker control material. The liver fibrosis marker control material is a lyophilized product prepared from matrix diluent, hyaluronic acid (HA), laminin (LN),type III procollagen (PIIINP), type IV collagen (Col IV) and glycocholic acid (CG). The control material prepared by the invention has the advantages of good uniformity and stability, free from influence of transportation, temperature and the like, suitability for quantitative detection of different instrument models and different reagents, as well as detection operation of different laboratory personnel in different regions. After the control material is redissolved, the detection process has a longer stable time, detection repeatability and high accuracy are realised; the quality control requirements of clinical detection of a liver fibrosis marker can be met and further the accuracy of clinical sample test results can be detected more accurately.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Micro-balloon injection for pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate
InactiveCN101822644AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsMicrospherePolyethylene glycol
The invention discloses a micro-balloon injection for a pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate, which is characterized by comprising cefmenoxime hydrochloride, anhydrous sodium carbonate, polylactic acid- polyethylene glycol block copolymer, poloxamer 188, glycerol and mannitol. The optimal scheme of the invention is the micro-balloon injection for the pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate, which is characterized by comprising 1 part of cefmenoxime hydrochloride, 0.12-0.18 part of anhydrous sodium carbonate, 1.2-4.5 parts of polylactic acid- polyethylene glycol block copolymer, 0.8-2 part(s) of poloxamer 188, 0.5-1 part of glycerol and 3-6 parts of mannitol. Compared with the prior art, the micro-balloon injection for the pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate prepared by the invention has high stability, the preparation process is simple and is suitable for industrial production, and has high encapsulation efficiency. As anhydrous sodium carbonate is used as a latent solvent, the re-dissolution is good, and has an appropriate slow-release effect.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation method of urchin-like nano gold particles and method for marking protein
ActiveCN107824800AEasy reunionEasy to reconstitutePeptide preparation methodsNanotechnologyEthanesulfonic acidGold particles
The invention discloses a preparation method of urchin-like nano gold particles. The preparation method is characterized by mainly comprising the following steps: adding a chloroauric acid solution into an N-(2-ethoxy) piperazine-N'-2-ethanesulfonic acid solution, carrying out full and uniform stirring and mixing, slowly stirring the solution till the solution turns from colorless transparency into a dark blue color, stopping stirring, stewing for 10 to 25 minutes to fully complete the reaction, and then adding citric acid or potassium carbonate to adjust the pH value to be 5.5 to 8.5. Compared with the prior art, the preparation method has the advantages of quickness, no need of gold seeds, green synthesis and the like.
Owner:SHIHEZI UNIVERSITY
Methods of enhancing solubility of agents
This invention concerns novel methods of enhancing the solubility of a compound. Compositions prepared using such methods are also disclosed. Compositions prepared using the methods have various advantages over conventionally known compositions.
Owner:FORMATECH
Novel coronavirus single-chain antibody, quality control product and preparation method
ActiveCN112538111AHigh sensitivityGood stabilityImmunoglobulins against virusesGenetic engineeringChemistryMolecular biology
The invention relates to a novel coronavirus single-chain antibody, a quality control product and a preparation method. The novel coronavirus single-chain antibody sequentially comprises a light chainvariable region and a heavy chain variable region from the N section to the C end, wherein an amino acid sequence of the light chain variable region is as shown in SEQ ID NO:1, and an amino acid sequence of the heavy chain variable region is as shown in SEQ ID NO:2. The novel coronavirus single-chain antibody is high in sensitivity, good in stability and very suitable for preparation of the quality control product. Moreover, on the basis of the sequences of the variable regions, a gene recombination technology is further used for constructing and forming a CH1 fragment-free human-mouse chimeric IgM antibody, so that the molecular stability and the specific binding capacity are improved. The prepared novel coronavirus single-chain antibody can be applied to a chemiluminescence platform forquality control product detection, the activity can be effectively determined, and the defects that a common 2019-nCoV IgM antibody is poor in detection sensitivity and low in specificity can be overcome.
Owner:SHENZHEN YHLO BIOTECH
Medical diagnosis reagent powder and its re-dissolving device and method
InactiveCN1847406AGuaranteed stabilityImprove stabilityMicrobiological testing/measurementBiological testingSemi automaticMedical diagnosis
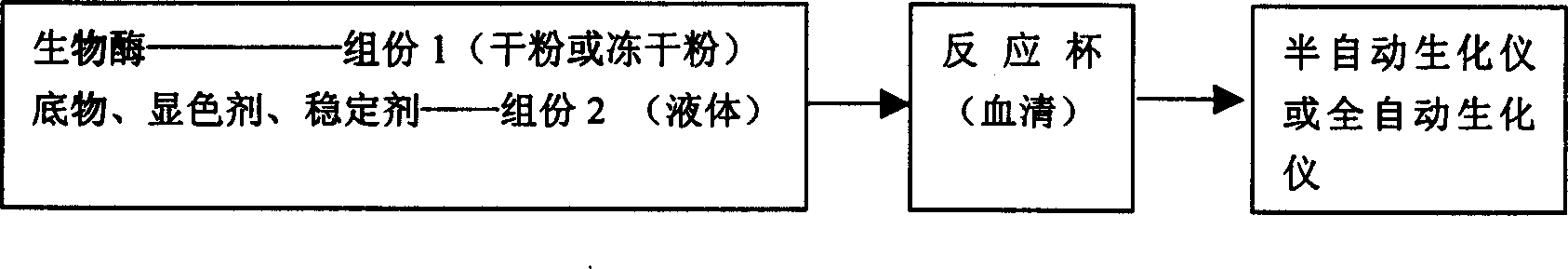
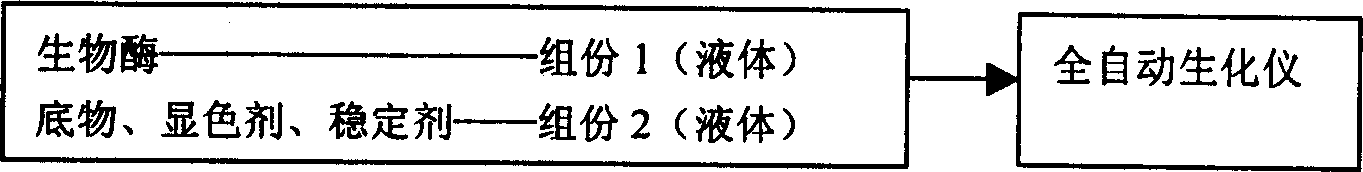
The medical diagnosis reagent powder re-dissolving device includes a liquid container, a powder container and a connection structure for mixing liquid or dissolving powder. The corresponding re-dissolving is also disclosed. The present invention can realize the inflow of liquid to the powder, the entering of powder into the liquid or the inflow of one kind of liquid to the other for mixing without need of opening the powder bottle lid and liquid bottle lid successively or simultaneously. The present invention can facilitate the measurement in spectrophotometer, semi-automatic biochemical analyzer and automatic biochemical analyzer, and has convenient operation, high efficiency, and high reagent stability.
Owner:朱小晖
TORCH-IgM antibody mixed quality control product
InactiveCN109254146AImprove uniformityImprove stabilityChemiluminescene/bioluminescenceFreeze-dryingQuality control
The invention discloses a TORCH-IgM antibody mixed quality control product, comprising a freeze dried product formed by a matrix diluent and five antibodies of toxoplasma gondii IgM, rubella virus IgM, cytomegalovirus IgM, type-1 herpes simplex virus IgM and type-2 herpes simplex virus IgM. The invention has the advantages that the prepared TORCH-IgM antibody mixed quality control product has gooduniformity and stability, is not influenced by transportation, temperature and other factors, has longer stabilization time in the detection process after being redissolved, has detection repeatability and high accuracy, can meet the quality control requirement of clinic on TORCH-IgM antibody detection, and can increase the accuracy of detection results of clinic samples.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Microsphere injection of medicinal composition of cefotiam hydrochloride and anhydrous sodium carbonate
InactiveCN101810584AImprove stabilityHigh encapsulation efficiencyOrganic active ingredientsDigestive systemCefotiam HydrochlorideMicrosphere
The invention discloses a microsphere injection of a medicinal composition of cefotiam hydrochloride and anhydrous sodium carbonate, which is characterized by consisting of the cefotiam hydrochloride, the anhydrous sodium carbonate, PLGA, fatty acid sorbitan-80, glycerol and sorbitol, in particular 1 part of the cefotiam hydrochloride, 0.15 to 0.2 part of the anhydrous sodium carbonate, 2.5 to 5 parts of PLGA, 1 to 3 parts of the fatty acid sorbitan-80, 0.8 to 1.5 parts of the glycerol and 5 to 10 parts of the sorbitol. Compared with the prior art, the microsphere injection of the medicinal composition of the cefotiam hydrochloride and the anhydrous sodium carbonate has the characteristics of high stability, high encapsulation rate and suitable slow-releasing effect, and a preparation process of the microsphere injection is suitable for industrial production.
Owner:HAINAN MEIDA PHARMA
Freeze-dried reagent manufacturing process for preventing deliquescence of freeze-dried reagent balls and freeze-dried reagent
InactiveCN112591160APrevent deliquescenceEasy to transportSolid materialLiquid materialPlastic packagingBiology
The invention relates to the field of freeze-dried preparations in the field of biological medicines, in particular to a freeze-dried reagent manufacturing process for preventing deliquescence of freeze-dried reagent balls. According to the manufacturing process, the surfaces of the freeze-dried reagent balls are coated with paraffin through a coating process, so that deliquescence of freeze-driedfinished products such as the freeze-dried reagent balls is effectively prevented, and meanwhile the beneficial effects of being convenient to transport, redissolve and store can be achieved. The problems that in the prior art, biological agents, namely the freeze-dried preparations or freeze-dried micro-cores and freeze-dried balls are extremely prone to deliquescence and inconvenient to store and transport are solved, and meanwhile the problems that in the using process, sealing materials such as bottles and plastic packages are adopted, inconvenience is brought in use, and the cost is highare solved.
Owner:SHANGHAI JANZY BIOTECHNOLOGY CO LTD
Process for preparing mezlocillin sodium by utilizing a solvent and crystallization
InactiveCN111471057ASimple production processAccurate measurementOrganic chemistry methodsProcess engineeringImidazolidinone
The invention discloses a process for preparing mezlocillin sodium by utilizing a solvent and crystallization. The process comprises firstly synthesizing 1-chloroformyl-2-imidazolidinone; then synthesizing 1-chloroformyl-3-methylsulfonyl-2-imidazolidinone and mezlocillin acid, and finally synthesizing the mezlocillin sodium. Starting from human basic raw materials, the production process of mezlocillin sodium is optimized, the obtained product is high in content, good in solubility and easy to redissolve, a series of problems in a freeze-drying process are solved, and further popularization ofthe product is facilitated.
Owner:JIANGSU HAIHONG PHARMA
Cefamandole nafate/anhydrous sodium carbonate medicinal composition microsphere injection
InactiveCN101836960AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsMicrosphereGlucose polymers
The invention discloses cefamandole nafate / anhydrous sodium carbonate medicinal composition microsphere injection, which is characterized by consisting of cefamandole nafate, anhydrous sodium carbonate, PGLA, Tween-80, propylene glycol and glucose. The invention adopts the preferable scheme that the cefamandole nafate / anhydrous sodium carbonate medicinal composition microsphere injection is characterized by comprising the following components in part by weight: 1 part of cefamandole nafate, 0.08 to 0.12 part of anhydrous sodium carbonate, 1.5 to 6 parts of PGLA, 0.2 to 2 parts of Tween-80, 0.3 to 3 parts of propylene glycol and 2 to 8 parts of glucose. Compared with the prior art, the prepared cefamandole nafate microspheres have stability, a preparation process is simple and suitable for industrialized production, the entrapment rate is high, and the anhydrous sodium carbonate used as a co-solvent has good resolution and proper slow-release effect.
Owner:HAINAN MEIDA PHARMA
A preparation method of sea urchin-shaped gold nanoparticles and a method for labeling proteins
ActiveCN107824800BEasy reunionEasy to reconstitutePeptide preparation methodsNanotechnologyGold particlesULTRAMARINE BLUE
The invention discloses a preparation method of urchin-like nano gold particles. The preparation method is characterized by mainly comprising the following steps: adding a chloroauric acid solution into an N-(2-ethoxy) piperazine-N'-2-ethanesulfonic acid solution, carrying out full and uniform stirring and mixing, slowly stirring the solution till the solution turns from colorless transparency into a dark blue color, stopping stirring, stewing for 10 to 25 minutes to fully complete the reaction, and then adding citric acid or potassium carbonate to adjust the pH value to be 5.5 to 8.5. Compared with the prior art, the preparation method has the advantages of quickness, no need of gold seeds, green synthesis and the like.
Owner:SHIHEZI UNIVERSITY
Application of natural polymeric polysaccharide in preparation of sample treatment solution and application of natural polymeric polysaccharide in immunochromatography detection kit
PendingCN113588943AReduce non-specific detection signalEasy to reconstituteImmunoassaysSpecific adsorptionSpecific detection
The invention discloses a new application of a natural polymeric polysaccharide Biosaccharide Gum <-1 >, and particularly relates to an application of the natural polymeric polysaccharide Biosaccharide Gum <-1 > in reduction of a non-specific signal of an in vitro diagnostic kit. The invention further provides a sample treatment solution containing the natural polymeric polysaccharide Biosaccharide Gum <-1 >. Biosaccharide Gum <-1 > in the treatment solution can enhance the resolubility of a fluorescent microsphere marker, and can be more easily released from a marking pad, in addition, Biosaccharide Gum <-1 > quickly forms a layer of linear polysaccharide film on an immune reaction coating film due to the film forming characteristic, the non-specific adsorption of the fluorescent microsphere marker on the coating film is reduced, further, the detection sensitivity of a fluorescence immunochromatography detection kit is improved, and meanwhile, the false positive of the kit is reduced. The sample treatment solution provided by the invention has the following advantages: the resolubility of the marker and the release capacity of the marker pad in the immunochromatography kit are improved; the non-specific detection signal of the kit is reduced, and the detection sensitivity of an immunochromatography reagent is improved; and the false positive of the kit is reduced.
Owner:BEIJING NORTH INST OF BIOLOGICAL TECH
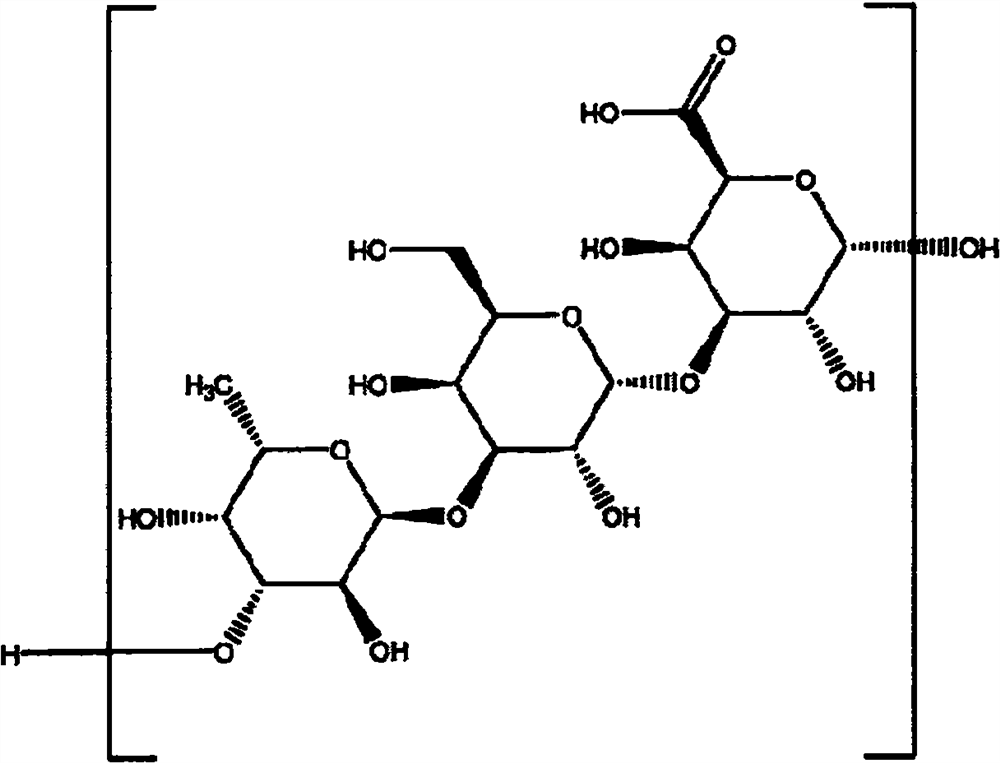
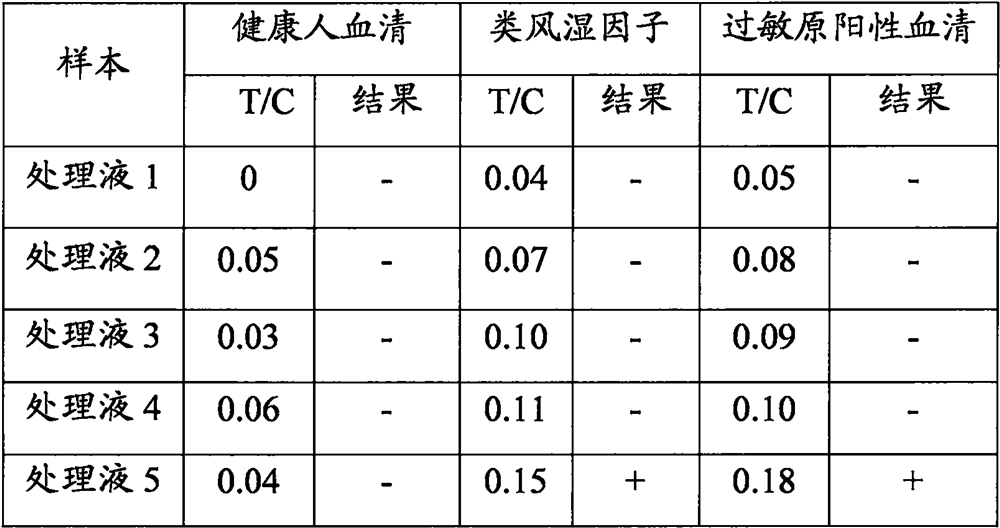
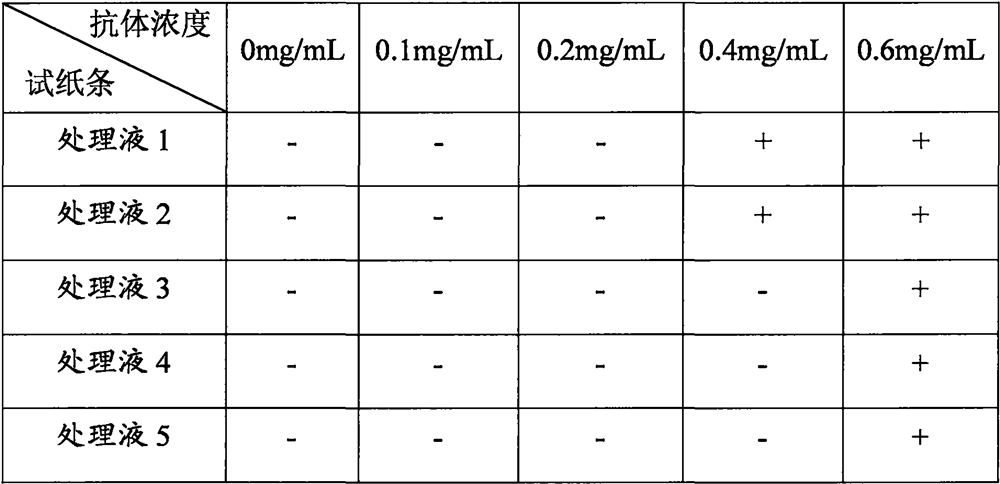
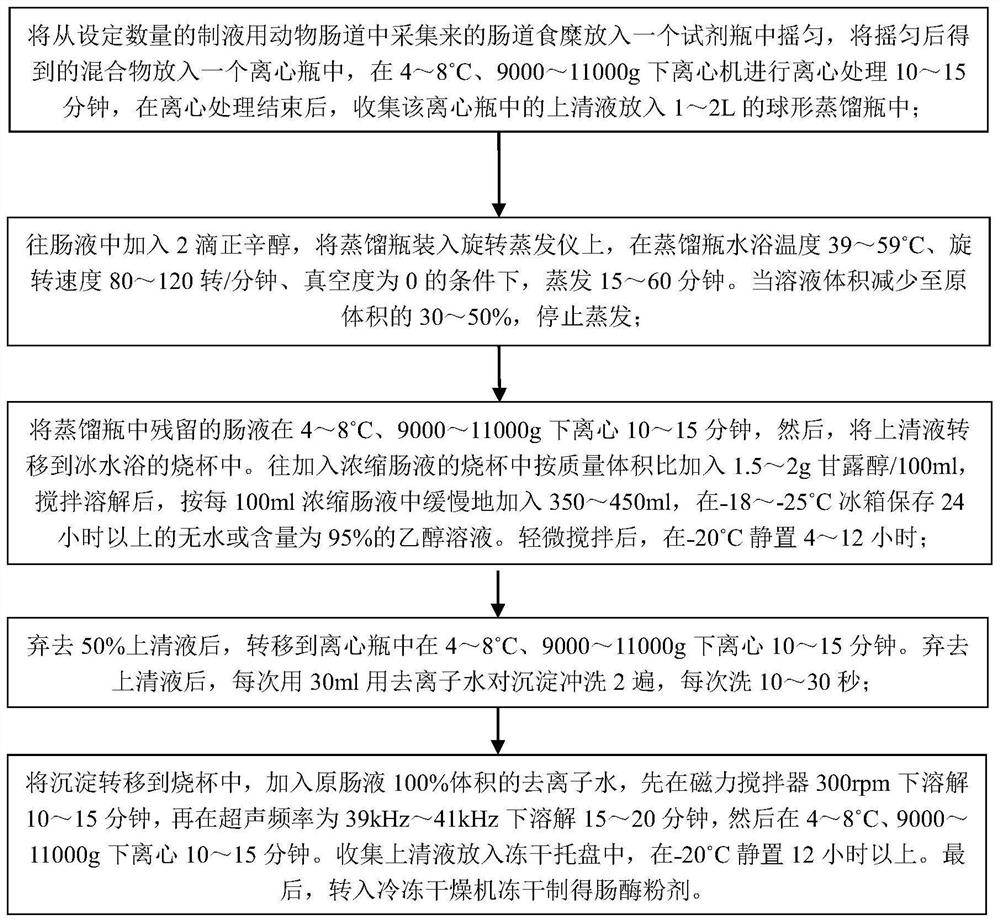
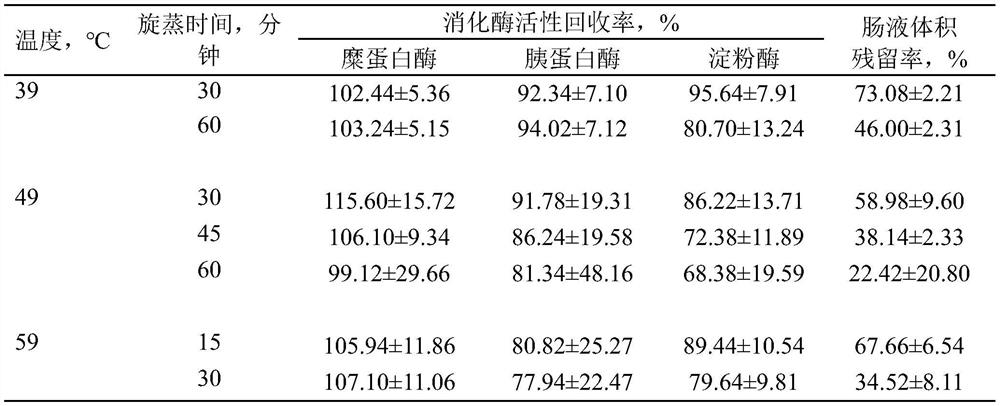
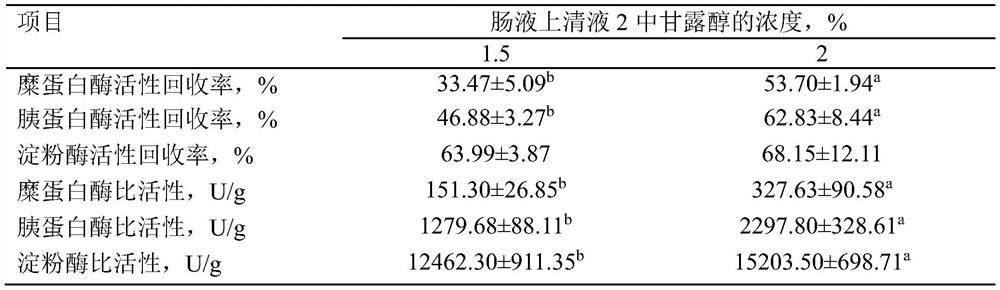
A kind of highly water-soluble porcine small intestine digestive enzyme powder and its preparation method and application
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Micro-balloon injection for pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate
InactiveCN101822644BImprove stabilityHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsMicrospherePolyethylene glycol
The invention discloses a micro-balloon injection for a pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate, which is characterized by comprising cefmenoxime hydrochloride, anhydrous sodium carbonate, polylactic acid- polyethylene glycol block copolymer, poloxamer 188, glycerol and mannitol. The optimal scheme of the invention is the micro-balloon injection for the pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate, which is characterized by comprising 1 part of cefmenoxime hydrochloride, 0.12-0.18 part of anhydrous sodium carbonate, 1.2-4.5 parts of polylactic acid- polyethylene glycol block copolymer, 0.8-2 part(s) of poloxamer 188, 0.5-1 part of glycerol and 3-6 parts of mannitol. Compared with the prior art, the micro-balloon injection for the pharmaceutical composition of cefmenoxime hydrochloride / anhydrous sodium carbonate prepared by the invention has high stability, the preparation process is simple and is suitable for industrial production, and has high encapsulation efficiency. As anhydrous sodium carbonate is used as a latent solvent, the re-dissolution is good, and has an appropriate slow-release effect.
Owner:HAINAN LINGKANG PHARMA CO LTD
New coronavirus single-chain antibody, quality control substance and preparation method
ActiveCN112538111BHigh sensitivityImprove stabilityImmunoglobulins against virusesFermentationIgm antibodyChimeric antibody
The present invention relates to a new coronavirus single-chain antibody, a quality control product and a preparation method, which include a light chain variable region and a heavy chain variable region in sequence from the N segment to the C terminal, and the amino acid sequence of the light chain variable region is as shown in SEQ As shown in ID NO:1, the amino acid sequence of the heavy chain variable region is shown in SEQ ID NO:2. The novel coronavirus single-chain antibody of the present invention has high sensitivity and good stability, and is very suitable for the preparation of quality control products. Moreover, on the basis of this variable region sequence, further use gene recombination technology to construct a human-mouse chimeric IgM antibody without a CH1 fragment, thereby improving molecular stability and specific binding ability. The novel coronavirus single-chain antibody prepared by the invention can be applied to a chemiluminescence platform for quality control detection, can effectively measure activity, and can overcome the defects of poor detection sensitivity and low specificity of general 2019‑nCoV IgM antibodies.
Owner:深圳市昭蓝生物科技有限公司
Flucloxacilin sodium liposome injection
InactiveCN102133181BImprove stabilityHigh encapsulation efficiencyAntibacterial agentsSolution deliveryFlucloxacillinGlycerol
The invention discloses a flucloxacilin sodium liposome injection. The flucloxacilin sodium liposome injection is mainly prepared from flucloxacilin sodium, polysorbate 80, gylcocholic acid sodium salt, gelatin, glycerol and sodium chloride; the defect of instability of the flucloxacilin preparation is overcome; and the liposome injection is prepared by protecting flucloxacilin sodium package by using liposome and then performing spray drying and aseptic subpackage, so that the stability of the flucloxacilin sodium is greatly increased, the preparation process is simple, yield is high, and solubility is high. The product quality of the preparation is improved and the toxic and side effects are reduced.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Flucloxacilin sodium liposome injection
InactiveCN102133181AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsSolution deliverySolubilityFlucloxacillin
The invention discloses a flucloxacilin sodium liposome injection. The flucloxacilin sodium liposome injection is mainly prepared from flucloxacilin sodium, polysorbate 80, gylcocholic acid sodium salt, gelatin, glycerol and sodium chloride; the defect of instability of the flucloxacilin preparation is overcome; and the liposome injection is prepared by protecting flucloxacilin sodium package by using liposome and then performing spray drying and aseptic subpackage, so that the stability of the flucloxacilin sodium is greatly increased, the preparation process is simple, yield is high, and solubility is high. The product quality of the preparation is improved and the toxic and side effects are reduced.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Non-refrigeration forming method and re-dissolving method for freeze-dried product of cosmetic raw material
ActiveCN112245330AAffectEasy to useCosmetic preparationsDrying solid materials without heatEthylene diamineFreeze-drying
The invention relates to the technical field of cosmetics, and for the problem that a liquid cosmetic raw material is easy to melt and is stuck on the wall after undergoing freeze forming to cause inconvenience of taking for use, discloses a non-refrigeration forming method and re-dissolving method for a freeze-dried product of a cosmetic raw material. The liquid cosmetic raw material to be treated is put in a mould with perforations, and is immersed in a coagulating bath and is shaken; after becoming spherical gel, the cosmetic raw material is taken out; the spherical gel is put in a packaging container for freeze drying after being washed with water; the cosmetic raw material contain sodium alginate, hyaluronic acid and hydrolyzed collagen; the coagulating bath is a calcium chloride aqueous solution; and an disodium ethylene diamine tetraacetic acid (EDTA) solution is adopted for redissolving. According to the forming method for the freeze-dried product of the cosmetic raw material,the situation that a part of cosmetic raw material melts after freeze forming to cause wall sticking in the freeze-drying process to influence use is avoided; the use efficiency of the mould is increased, and the continuous production process is easier to realize. The redissolving process of the freeze-dried product is safe and convenient, and cosmetic raw material is convenient to use.
Owner:杭州华玮生物科技有限公司
A kind of medicinal composition of vidarabine monophosphate for injection
ActiveCN103599080BFlat surfaceNot crackedPowder deliveryOrganic active ingredientsMedicineActive component
The invention relates to an officinal composition of vidarabine monophosphate for injection, wherein the main active components of the composition comprise vidarabine monophosphate and lysine.
Owner:GUANGDONG LONGFU MEDICINE CO LTD
Cefamandole nafate/anhydrous sodium carbonate medicinal composition microsphere injection
InactiveCN101836960BImprove stabilityHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsMicrosphereGlucose polymers
The invention discloses cefamandole nafate / anhydrous sodium carbonate medicinal composition microsphere injection, which is characterized by consisting of cefamandole nafate, anhydrous sodium carbonate, PGLA, Tween-80, propylene glycol and glucose. The invention adopts the preferable scheme that the cefamandole nafate / anhydrous sodium carbonate medicinal composition microsphere injection is characterized by comprising the following components in part by weight: 1 part of cefamandole nafate, 0.08 to 0.12 part of anhydrous sodium carbonate, 1.5 to 6 parts of PGLA, 0.2 to 2 parts of Tween-80, 0.3 to 3 parts of propylene glycol and 2 to 8 parts of glucose. Compared with the prior art, the prepared cefamandole nafate microspheres have stability, a preparation process is simple and suitable forindustrialized production, the entrapment rate is high, and the anhydrous sodium carbonate used as a co-solvent has good resolution and proper slow-release effect.
Owner:HAINAN MEIDA PHARMA
Freeze-dried preparation production process, freeze-dried preparation and freeze-dried reagent preparation box
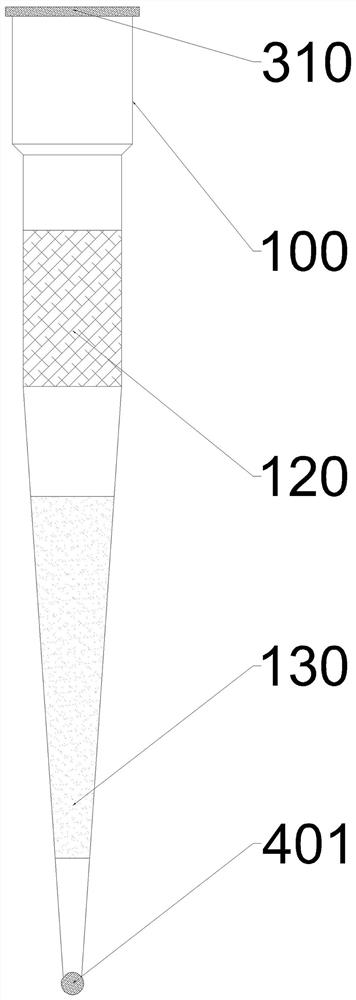
ActiveCN112237949AImprove general performanceEasy to get materialsDrying solid materials without heatBurettes/pipettesCryopreservationLiquid tank
The invention relates to the technical field of freeze-drying of biological reagents, in particular to aFreeze-dried preparation production process. A pipettor tip is used as a carrier of the Freeze-dried preparation. The pipettor suction head sucks the stock solution and then directly carries out cryopreservation in the pipettor suction head to generate a freeze-dried reagent, and then packagingis carried out. When in use, the pipette is directly connected. The invention also discloses aFreeze-dried reagent preparation box based on the pipettor sucker as a carrier of a lyophilized preparation. The Freeze-dried reagent preparation box comprises a shell, the pipettor sucker and a reconstitution fluid carrier. The shell is provided with a cavity with an open upper part; a freeze-dried reagent is placed at the bottom of the pipettor sucker; the reconstitution fluid carrier is provided with a liquid tank, a liquid tank film is arranged at the upper part of the liquid tank, and a reconstitution fluid is stored in the reconstitution fluid carrier; the pipettor sucker is positioned at the upper part of the reconstitution fluid carrier, and the tip faces the liquid tank film; and a sealing piece is arranged at the upper opening of the cavity of the shell. The invention is convenient for storage and transportation of freeze-dried reagents, is convenient for redissolving of the freeze-dried reagents, and avoids pollution in the redissolving process.
Owner:SHANGHAI JANZY BIOTECHNOLOGY CO LTD
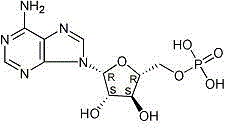
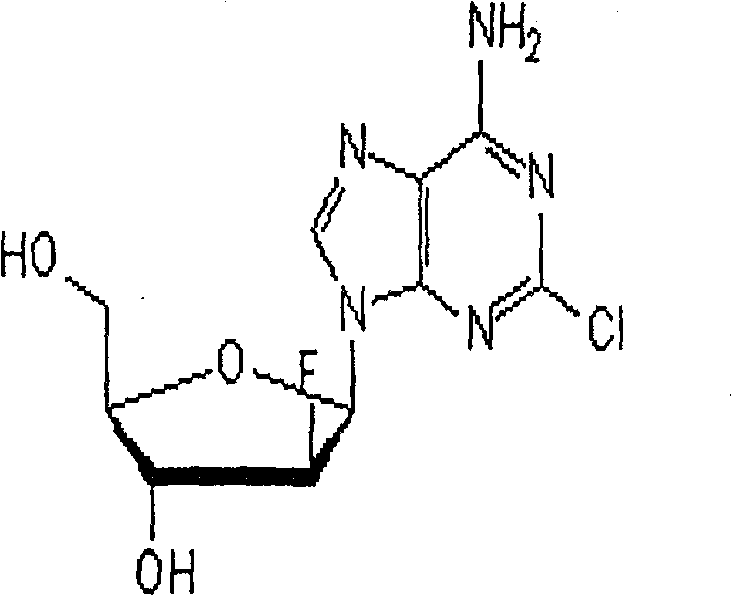
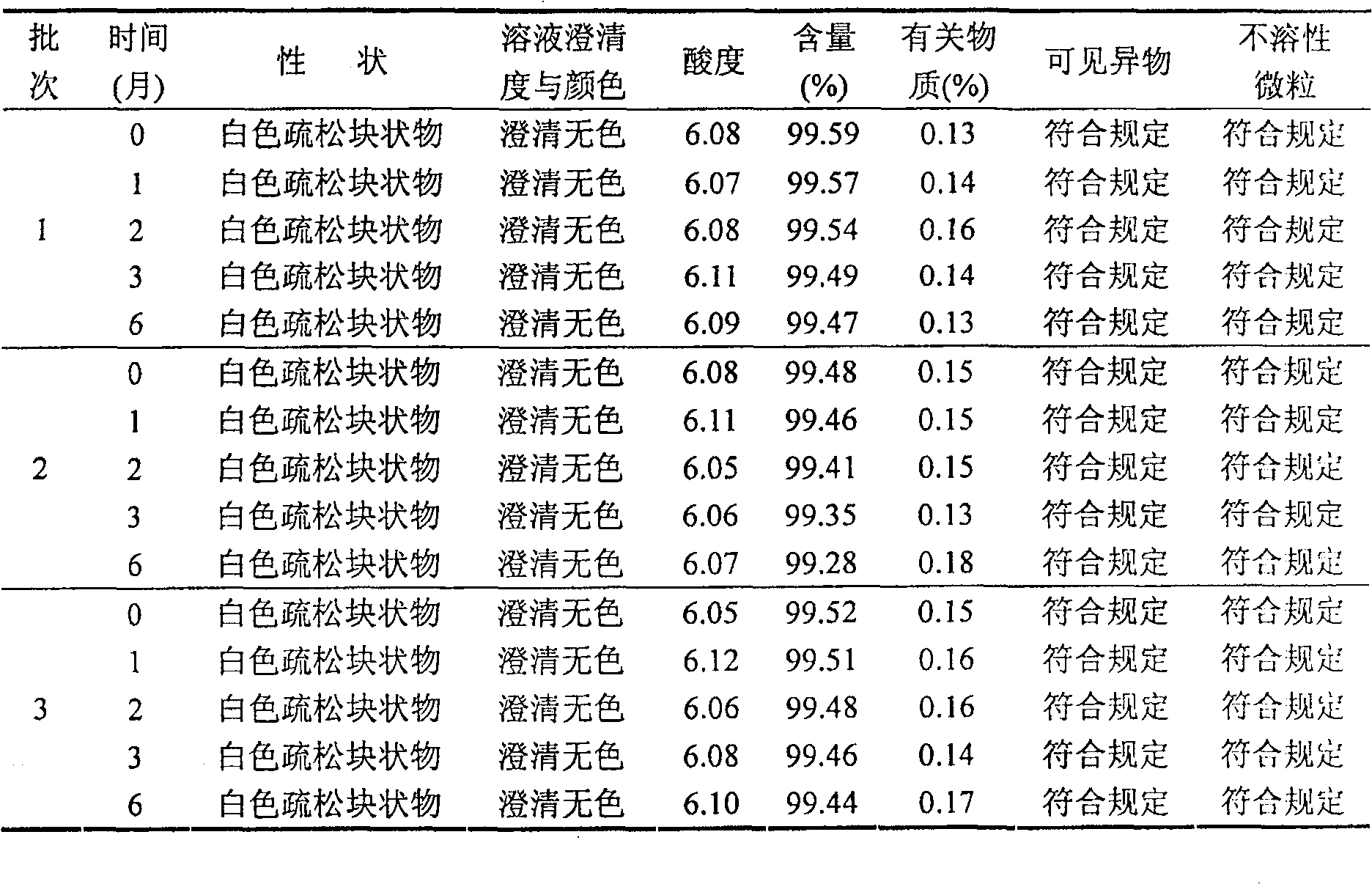
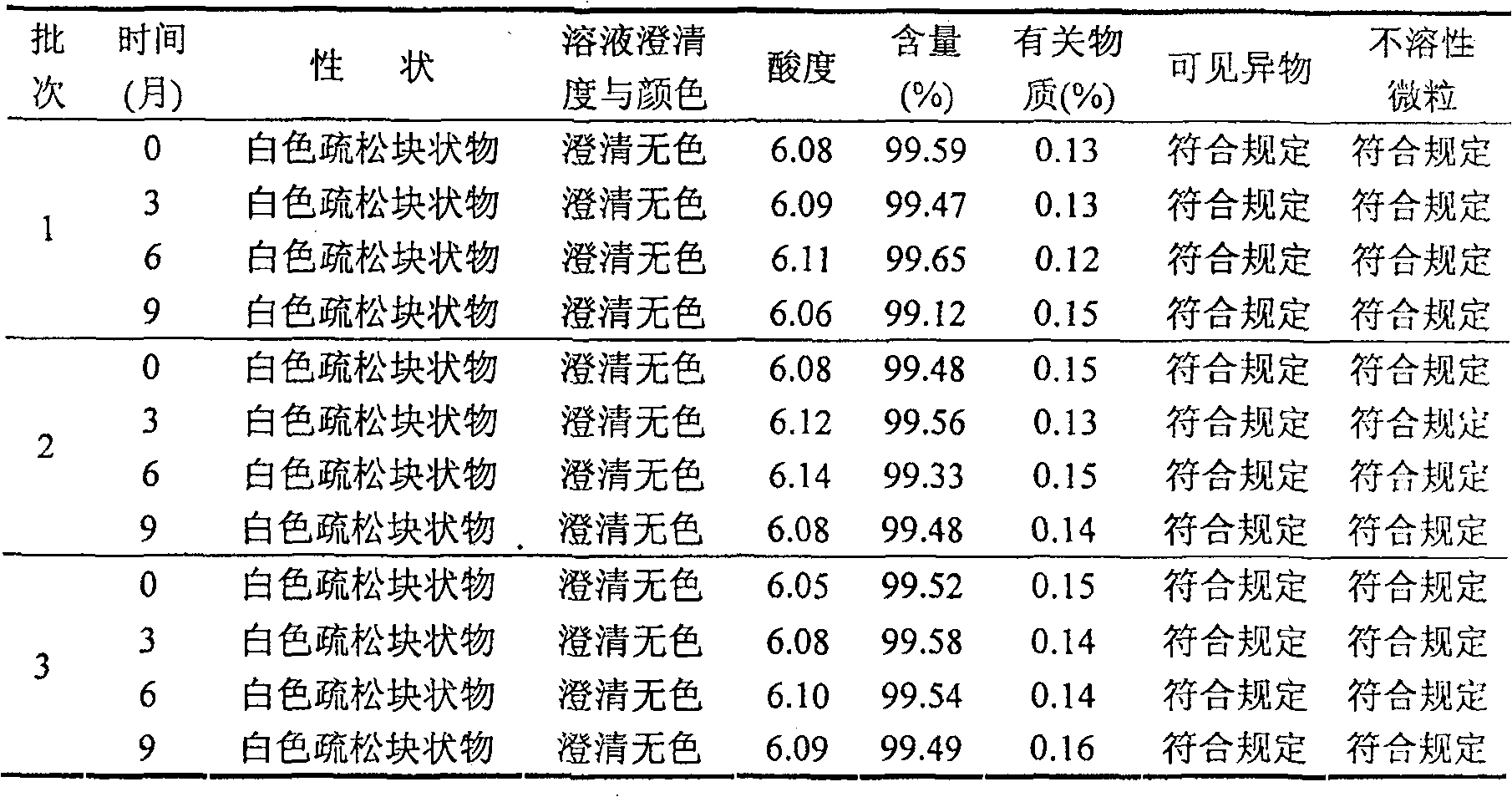
Freezing-dried clofarabine powder injection and its preparation method
InactiveCN100591330CLow content of related substancesEasy to storeOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLMedicine
The present invention relates to a Clofarabine freeze drying powder and the preparation method, which comprises Clofarabine and at least one biological acceptable excipient. Wight ratio of the Clofarabine and the excipient is from one to 10 till one to 50. The excipient is selected from one kind of mannitol and lactose. The optional choice is the mannitol. The preparation method is as following. The Clofarabine is melted with the injection water and the excipient is added. Followed by well mixing , filtration, packaging, adding stopper and loading in the plate. The freeze dryer is opened in advance. The platelayer is cooled with the heat conduction oil until temperature of the platelayer increases till minus 40 DEG Cto minus 50 DEG C. The Clofarabine solution packaged in the sterile silinbottle can be sent to the freeze dryer quickly.The box door is shut and the plate temperature is kept at minus 40 DEG Cto minus 50 DEG C and the box temperature is decreased. When the sample temperature reaches minus 40 DEG C, The plate cooling is stopped and the condenser is opened. At the same time the product temperature is maintaineed by cooling for 3 hours. When the temperature of the condenser reaches minus 40 DEG, the vacuum system is opened. After temperature holding, four steps of heating, sublimation, drying, plugging, box pressing and opening sealing can be started.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com