Sperm chromatin co-immunoprecipitation method
A technology of co-immunoprecipitation and chromatin, applied in the field of sperm chromatin co-immunoprecipitation, can solve problems such as low efficiency, and achieve the effect of reducing difficulty, improving digestion efficiency and improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Take human sperm (the number is about 2×10) frozen at -80°C 7 ), adding paraformaldehyde (PFA) with a mass concentration of 0.5%, and fixing at 22° C. for 10 min.
[0057] 2. Centrifuge at 5,000g at 22°C for 10 minutes, and discard the supernatant.
[0058] 3. Add 4°C pre-cooled PBS to wash once, centrifuge at 5,000g at 22°C for 10 minutes, and discard the supernatant.
[0059] 4. Add 300 μl of lysis solution (0.5% SDS, 10 mM DTT) to the sperm pellet, and incubate at 22° C. for 1 hr.
[0060] 5. Then add 3 μl of 1M N-ethylmaleimide to terminate the effect of DTT.
[0061] 6. Then use ultrasonic treatment, ultrasonic 30s (choose Low power of the ultrasonic device), interval 45s, 10 cycles; use micrococcal nuclease to digest after ultrasonication, the enzyme addition is 200U / 10 6 spermatozoa, the enzymolysis temperature was 37°C, and the enzymolysis time was 30 minutes to obtain digestive juice.
[0062] 7. Centrifuge the above digestive solution at 12,000rpm for 1...
Embodiment 2
[0098] Human sperm was replaced with mouse sperm, and the rest of the operations were the same as in Example 1.
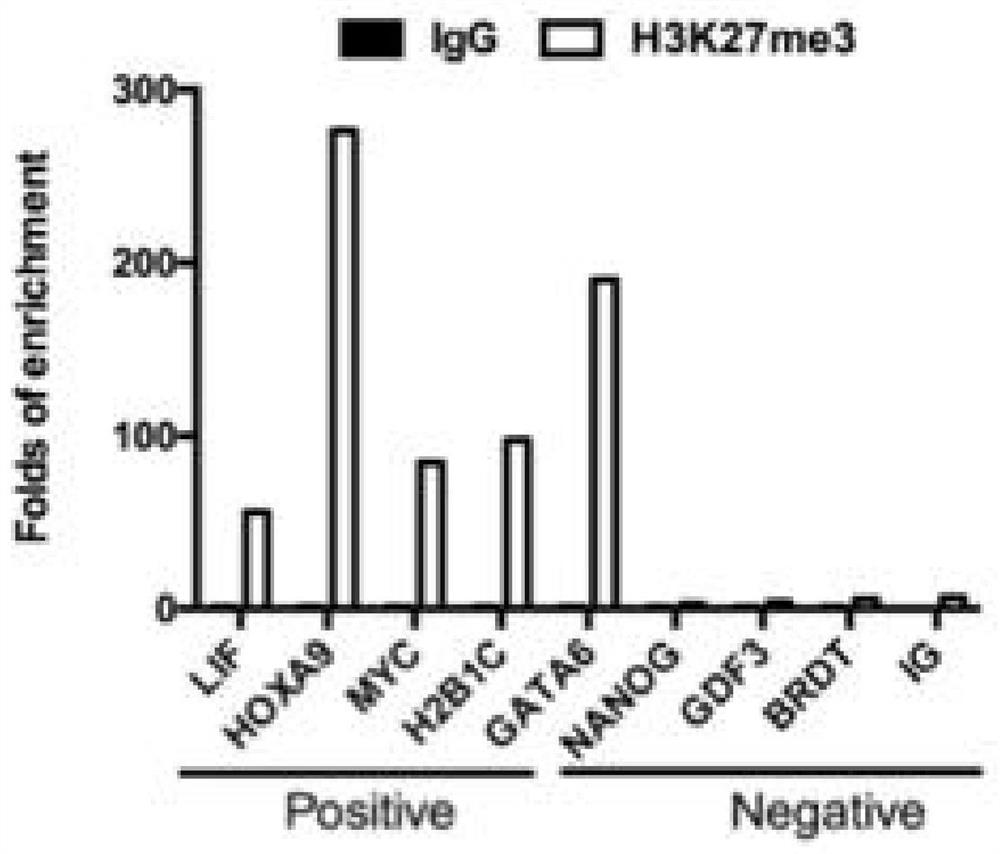
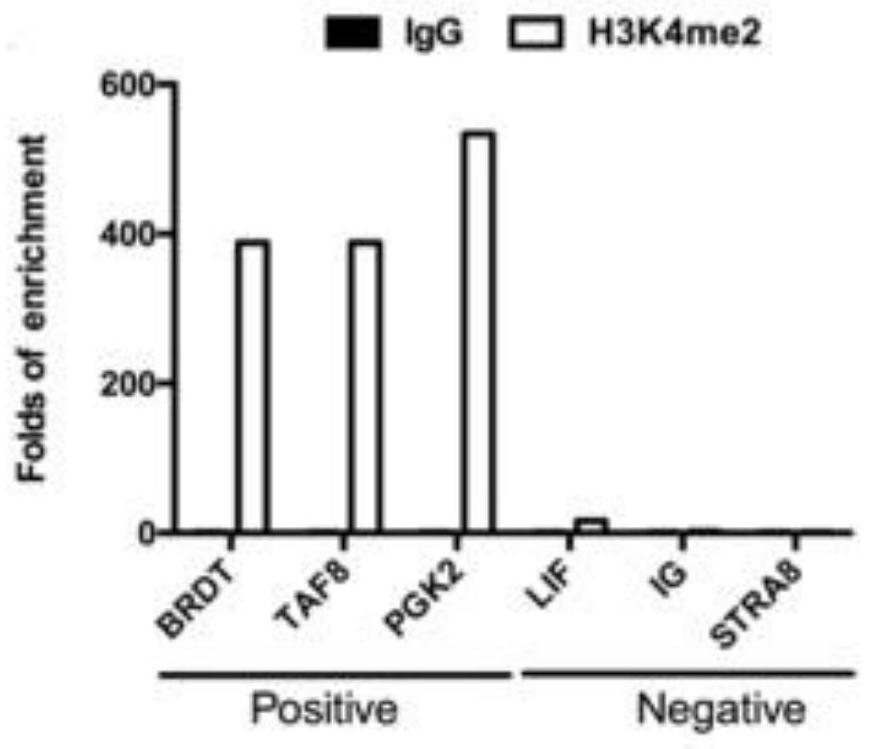
[0099] Validation of H3K4me2 and H3K27me3 positive and negative sites as Figure 4 , Figure 5 shown.
Embodiment 3
[0101] Optimize the ultrasonic conditions, change the number of ultrasonic cycles to 0, 3, 6, and 10, and then perform agarose gel electrophoresis on the supernatant and precipitate of the digestive solution. The results are as follows: Image 6 shown.
[0102] The supernatant and precipitate after digestion and co-immunoprecipitation under the above conditions were used to calculate the DNA yield, and the results are shown in Table 2.
[0103] Table 2 DNA yield after optimization of ultrasonic conditions
[0104] sample Concentration (ng / μl) 0-S 254.4 0-P 342.4 3-S 361.5 3-P 52.9 6-S 210.6 6-P 91.2 10-S 106.2 10-P 48.0
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com