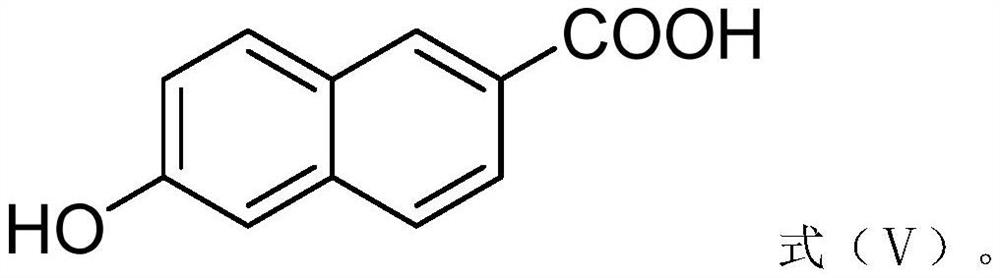
Preparation method of 6-hydroxy-2-naphthoic acid
A technology of naphthoic acid and sodium naphthoic acid, applied in the field of chemical technology, can solve the problems of poor conversion rate and selectivity, harsh reaction conditions and high equipment requirements, and achieve the effects of mild preparation conditions, low production cost and simple equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method of 6-hydroxyl-2-naphthoic acid, the steps are as follows:
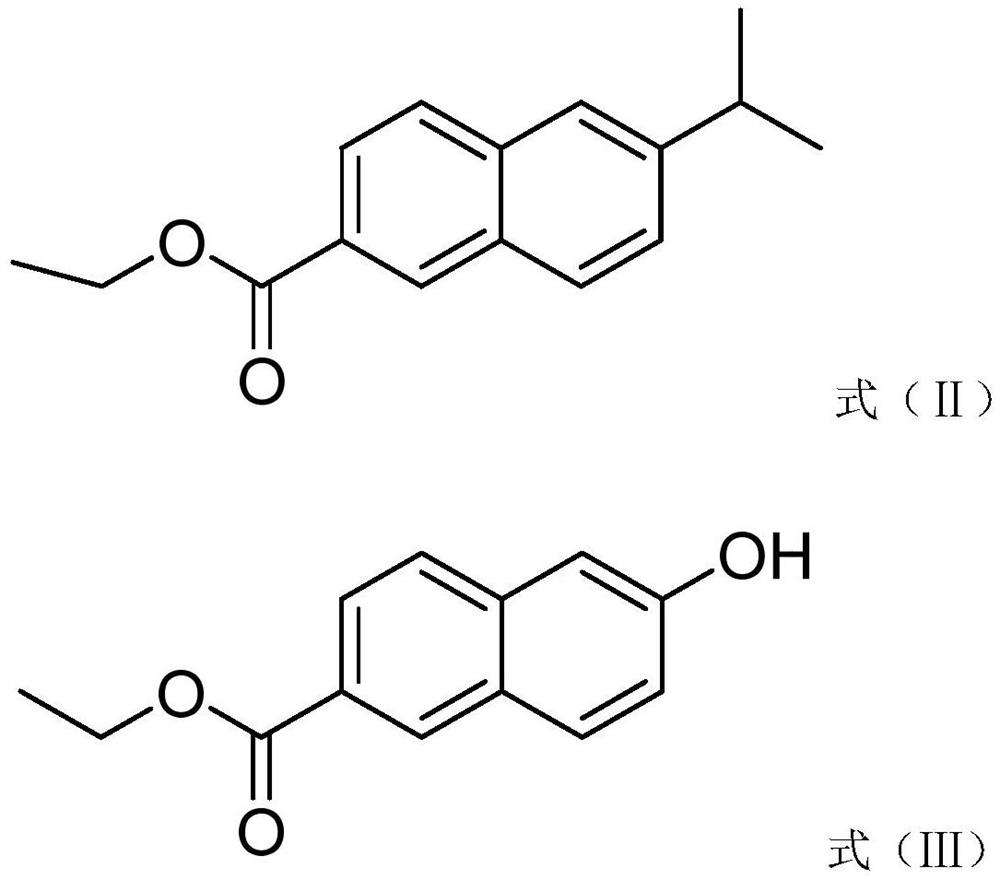
[0032] Synthesis of S1, ethyl 6-hydroxy-2-naphthoate: 22.8g ethyl 6-isopropyl-2-naphthoate, 1.6g N-hydroxymethylphthalimide, 2.0g azo Diisoheptanonitrile and 150 mL of ethyl acetate were placed in a 500 mL single necked round bottom flask fitted with a balloon filled with oxygen. The mixture was stirred at 45°C for 10 hours. The reaction mixture was treated with dilute sulfuric acid solution at room temperature for 0.6 hours. The solvent was removed under reduced pressure to obtain a crude mixture, which was purified by silica gel column chromatography to obtain the product, 13.3 g of ethyl 6-hydroxy-2-naphthoate, with a yield of 66%;
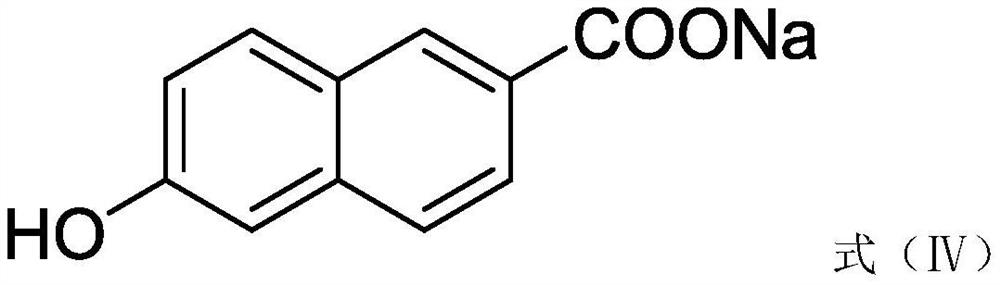
[0033] S2. Synthesis of sodium 6-hydroxy-2-naphthoate: 20.2 g of ethyl 6-hydroxy-2-naphthoate and 50 mL of 0.2 g / mL sodium hydroxide aqueous solution were added to a single-necked round-bottomed flask, and stirred at 36 ° C for 10 hours to obtain Sodium 6...
Embodiment 2
[0036] A preparation method of 6-hydroxyl-2-naphthoic acid, the steps are as follows:
[0037] Synthesis of S1, ethyl 6-hydroxy-2-naphthoate: 22.8g ethyl 6-isopropyl-2-naphthoate, 1.6g N-hydroxymethylphthalimide, 2.0g azo Dimethyl diisobutyrate and 150 mL m-xylene were placed in a 500 mL single necked round bottom flask fitted with a balloon filled with oxygen. The mixture was stirred at 50°C for 10 hours. The reaction mixture was treated with dilute hydrochloric acid solution at room temperature for 0.8 hours. The solvent was removed under reduced pressure to obtain a crude mixture, which was purified by silica gel column chromatography to obtain the product, 16.1 g of ethyl 6-hydroxy-2-naphthoate, with a yield of 88%;
[0038] S2. Synthesis of sodium 6-hydroxy-2-naphthoate: 20.2 g of ethyl 6-hydroxy-2-naphthoate and 50 mL of 0.2 g / mL potassium hydroxide aqueous solution were added to a single-necked round-bottomed flask, stirred at 38 ° C for 10.5 hours, Obtain sodium 6-h...
Embodiment 3
[0041] A preparation method of 6-hydroxyl-2-naphthoic acid, the steps are as follows:
[0042] Synthesis of S1, ethyl 6-hydroxy-2-naphthoate: 22.8g ethyl 6-isopropyl-2-naphthoate, 1.6g N-hydroxymethylphthalimide, 2.0g azo Dimethyl diisobutyrate and 150 mL m-xylene were placed in a 500 mL single necked round bottom flask fitted with a balloon filled with oxygen. The mixture was stirred at 50°C for 10 hours. The reaction mixture was treated with dilute hydrochloric acid solution at room temperature for 0.9 hours. The solvent was removed under reduced pressure to obtain a crude mixture, which was purified by silica gel column chromatography to obtain the product, 16.1 g of ethyl 6-hydroxy-2-naphthoate, with a yield of 88%;
[0043] S2. Synthesis of sodium 6-hydroxy-2-naphthoate: 20.2 g of ethyl 6-hydroxy-2-naphthoate and 50 mL of 0.2 g / mL potassium carbonate aqueous solution were added to a single-necked round-bottomed flask, and stirred at 39 ° C for 11 hours to obtain 6 -Sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com