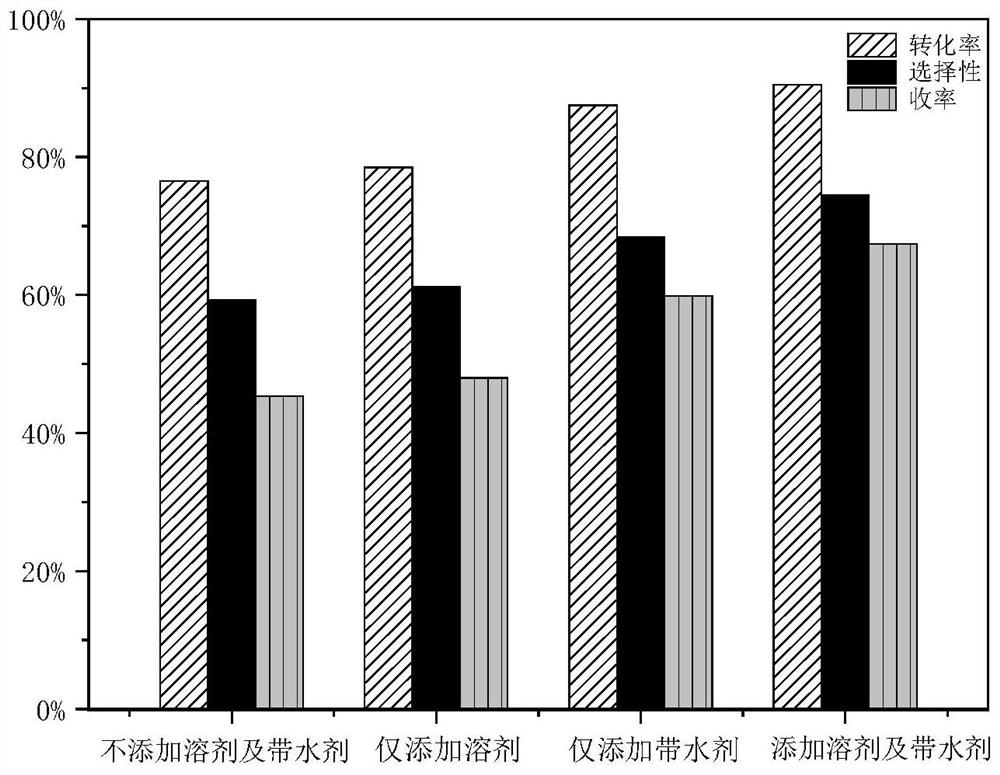
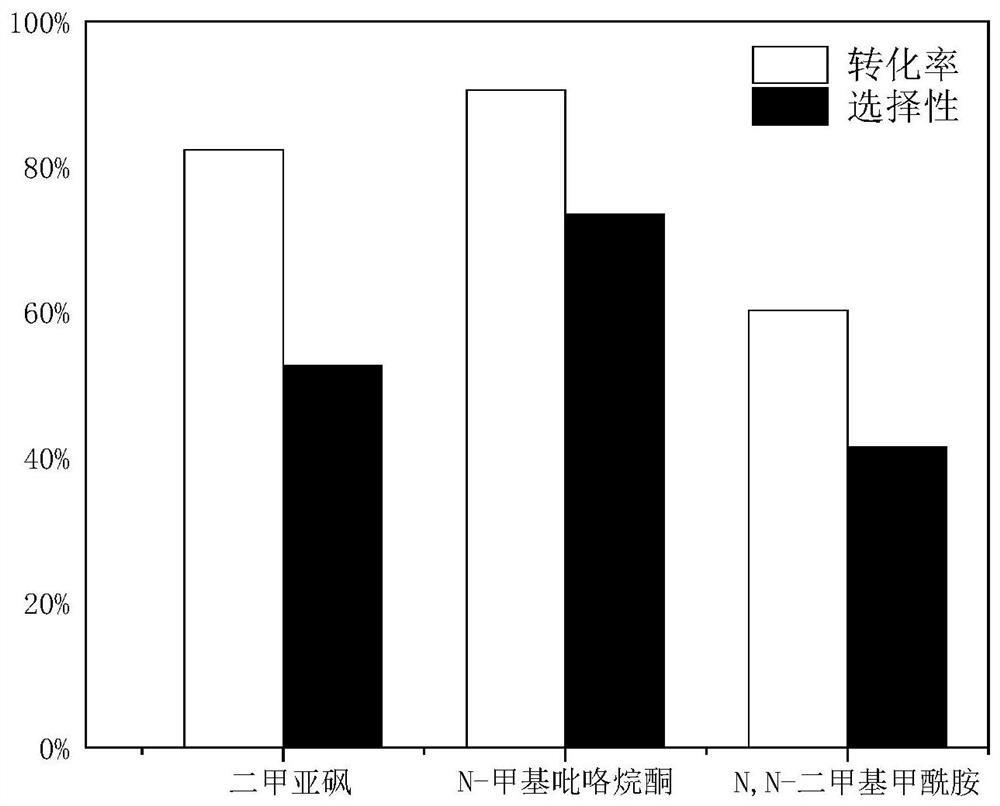
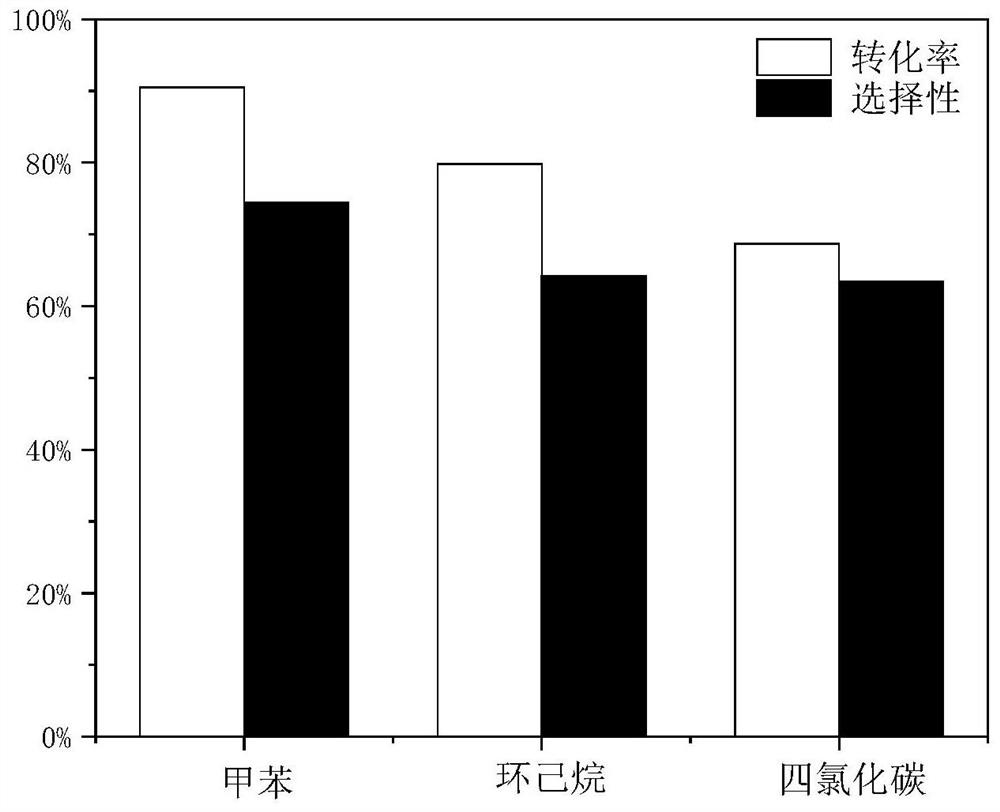
Method for preparing glyceryl oleate through reaction azeotropic distillation
A technology of azeotropic distillation of oleic acid glyceride, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of the incompatibility of oleic acid and glycerin as the raw material of the reaction, and achieve the improvement of immiscibility problems, ease of separation, and the effect of increasing the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add raw materials 3.68g glycerin, 33.8g oleic acid, 18.41g solvent N-methylpyrrolidone and 20g water-carrying agent toluene into the reaction kettle, then weigh 0.75g solid acid catalyst SO 4 2- / ZrO 2 -Al 2 o 3 , the reaction temperature was controlled at 190°C, and the reaction was stirred and rectified for 6h. After the reaction, the product was filtered to separate the catalyst, the solvent N-methylpyrrolidone was removed by washing with water, and the water-carrying agent toluene was removed by rotary evaporation under reduced pressure to obtain a crude glyceryl oleate product.
[0022] The solid acid catalyst SO of the present embodiment 4 2- / ZrO 2 -Al 2 o 3 The preparation method is as follows:
[0023] Dissolve zirconium oxychloride and aluminum nitrate at a molar ratio of 3:1 in deionized water. After dissolving evenly, stir vigorously at room temperature and drop 28% NH 3 ·H 2 O produces a white precipitate. After adjusting the pH of the system to ...
Embodiment 2
[0027] The basic steps are the same as in Example 1, except that the reaction temperature is different. The effects of each reaction temperature on the conversion of glycerol and the selectivity of glycerol oleate are shown in Table 1.
[0028] The reaction temperature of table 1 is to the conversion rate and the selectivity influence of synthetic oleic acid glyceride
[0029] Reaction temperature (°C) Conversion rates(%) selectivity (%) Yield (%) 140.0 59.5 25.0 14.9 150.0 65.4 27.3 17.9 160.0 74.4 28.1 20.9 170.0 83.8 32.7 27.4 180.0 86.2 34.8 30.0 190.0 89.7 37.6 33.7 200.0 90.1 36.5 32.9
[0030] It can be seen from Table 1 that different reaction temperatures have a greater impact on the conversion rate of the olein synthesis process, but a lesser impact on the selectivity. Raising the reaction temperature can speed up the reaction rate and increase the reaction conversion rate. However, when the react...
Embodiment 3
[0032] The preparation steps of glyceryl oleate in this embodiment are the same as those in Example 1, except that the alkyd molar ratio is different. The effect of each alkyd molar ratio on the conversion rate of glycerol and the selectivity of glycerol oleate is shown in Table 2.
[0033] Table 2 Alkyd molar ratio on the conversion rate and selectivity of synthetic olein glycerides
[0034] Alkyd ratio Conversion rates(%) selectivity (%) Yield (%) 1:3.01 89.7 37.6 33.7 1:3.22 91.3 46.5 42.5 1:3.39 89.8 54.1 48.6 1:3.61 90.9 67.5 61.4 1:3.82 90.8 73.0 66.3 1:4.00 90.5 74.5 67.4 1:5.00 90.3 72.7 65.6
[0035] It can be seen from Table 2 that when the molar ratio of alkyd to acid is changed, the conversion of glycerol hardly changes, but the product selectivity is significantly improved. The esterification reaction of oleic acid and glycerin includes the reaction of glycerin and oleic acid to form monooleate,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com