Hyperbranched polyamine carbon dioxide absorbent as well as preparation method and application thereof
A carbon dioxide and absorbent technology, applied in chemical instruments and methods, separation methods, reagents, etc., can solve the problems of insufficient carbon dioxide absorption capacity, low absorbent viscosity, slow absorption speed, etc., and achieve simple structure, high nitrogen content, Fast absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
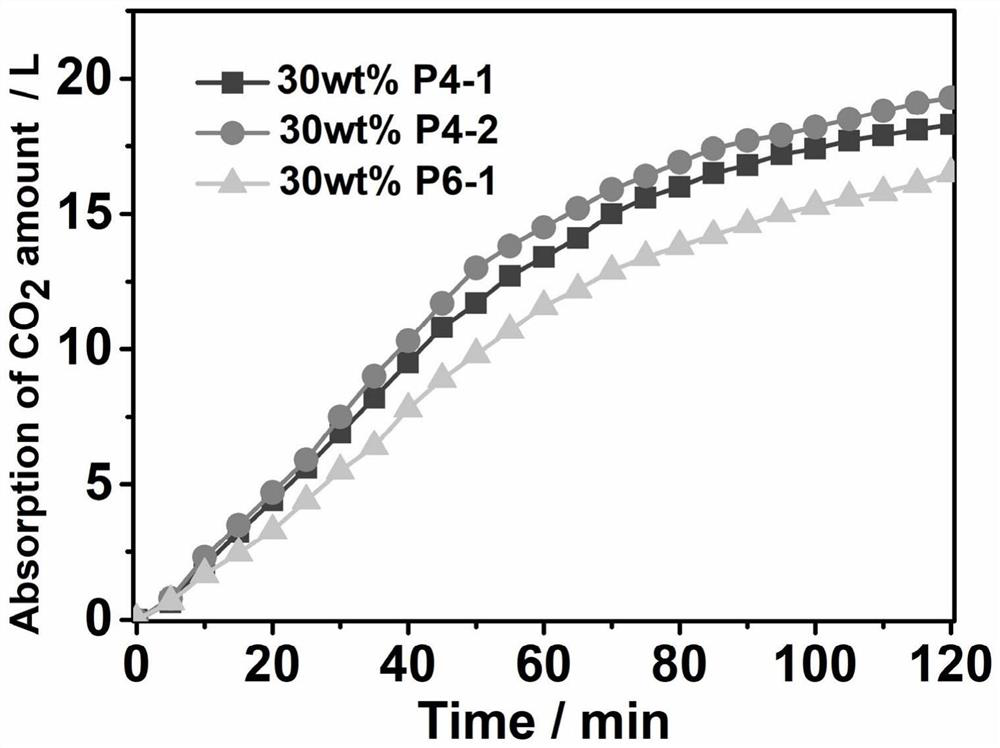
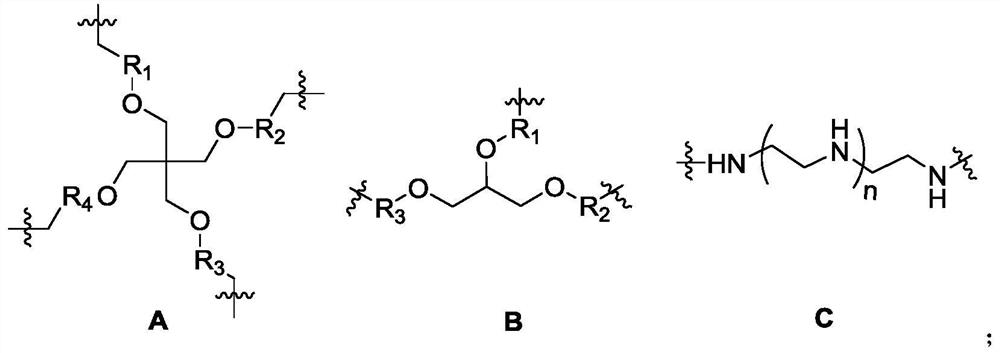
[0063] has the general formula -A-C- repeating unit, and Preparation of compound P4-1 with n=2;
[0064] The structural formula of P4-1 is:
[0065]
[0066] 1) Pentaerythritol (13.6 g, 0.1 mol) was dissolved in dry N,N-dimethylformamide (200 mL), and phosphorus tribromide (133 g, 0.5 mol) was added in batches at 25°C. After stirring for 20 minutes, it was slowly raised to 125°C for 12 hours of reaction. After the reaction, the reaction solution was poured into ice water (500mL), and sodium hydroxide solution (0.2mol L -1 ) to adjust the pH to 8, and the residue was suction-filtered to obtain tetrabromopentaerythyl alcohol (24.6 g, 64%). 1 H NMR (CDCl 3 ,400MHz):δ3.20(s,8H). 13 C NMR (CDCl 3 ,100MHz):δ146.1,37.9.HR-MS(MALDI):m / z[M] + cacld for C5H8Br4, 383.7860; found, 387.7319.
[0067] 2) Tetrabromopentaerythyl alcohol (19.2g, 0.05mol) and 4-hydroxybenzaldehyde (26.8g, 0.220mol) prepared in step 1), and potassium carbonate (41.4g, 0.30mol) were added to N,N- In ...
Embodiment 2
[0070] has the general formula -A-C- repeating unit, and Preparation of compound P4-2 with n=3;
[0071] The structural formula of P4-2 is:
[0072]
[0073] 1) Tetrabromopentaerythyl alcohol (19.2g, 0.05mol) and 3-hydroxybenzaldehyde (26.8g, 0.220mol), potassium carbonate (41.4g, 0.30mol) are added to N,N-dimethylformamide ( 250 mL), the temperature was raised to 100°C for 16 hours. The reaction solution was poured into ice water (500 mL), and the residue was suction-filtered to obtain intermediate product A-2 (20.9 g, 76%). 1 H NMR (CDCl 3 ,400MHz):δ3.823(s,8H),7.22(d,J=8.0Hz,4H),7.35(s,4H),7.56(d,J=8.0Hz,4H),7.65-7.72(m, 4H), 9.82(s, 4H). 13 C NMR (CDCl 3 ,100MHz):δ191.08,165.63,137.52,129.86,121.54,12.78,115.97,61.23,40.56.HR-MS(MALDI):m / z[M] + cacld for C33H28O8, 552.1784; found, 553.1786.
[0074] 2) The intermediate product A-2 (27.6g, 0.05mol) and C-2 (n=3) tetraethylenepentamine (19.4g, 0.10mol) prepared in step 1) were added to methanol (100mL), and At 0...
Embodiment 3
[0076] has the general formula -B-C- repeating unit, and The preparation of the compound P6-1 of n=2 The structural formula of P6-1 is:
[0077]
[0078] 1) Glycerol (9.2 g, 0.1 mol) was dissolved in dry N,N-dimethylformamide (200 mL), and phosphorus tribromide (106.4 g, 0.4 mol) was added in batches at 25°C. After stirring for 20 minutes, it was slowly raised to 120°C for 12 hours of reaction. After the reaction, the reaction solution was poured into ice water (500mL), and sodium hydroxide solution (0.2mol L -1 ) to adjust the pH to 8, and the residue was suction filtered to obtain tribromoglycerol (17.6 g, 63%). 1 H NMR (CDCl 3 ,400MHz): δ4.60-4.65(m,1H),3.74(d,J=8.0Hz 4H). 13 CNMR (CDCl 3 ,100MHz):δ48.9,34.7.HR-MS(MALDI):m / z[M] + cacld for C3H5Br3, 277.7941; found, 279.7921.
[0079] 2) Tribromoglycerol (13.9g, 0.05mol) and 4-hydroxybenzaldehyde (19.9g, 0.16mol) prepared in step 1), and potassium carbonate (27.6g, 0.20mol) were added to N,N- In dimethylformamide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| shear viscosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com