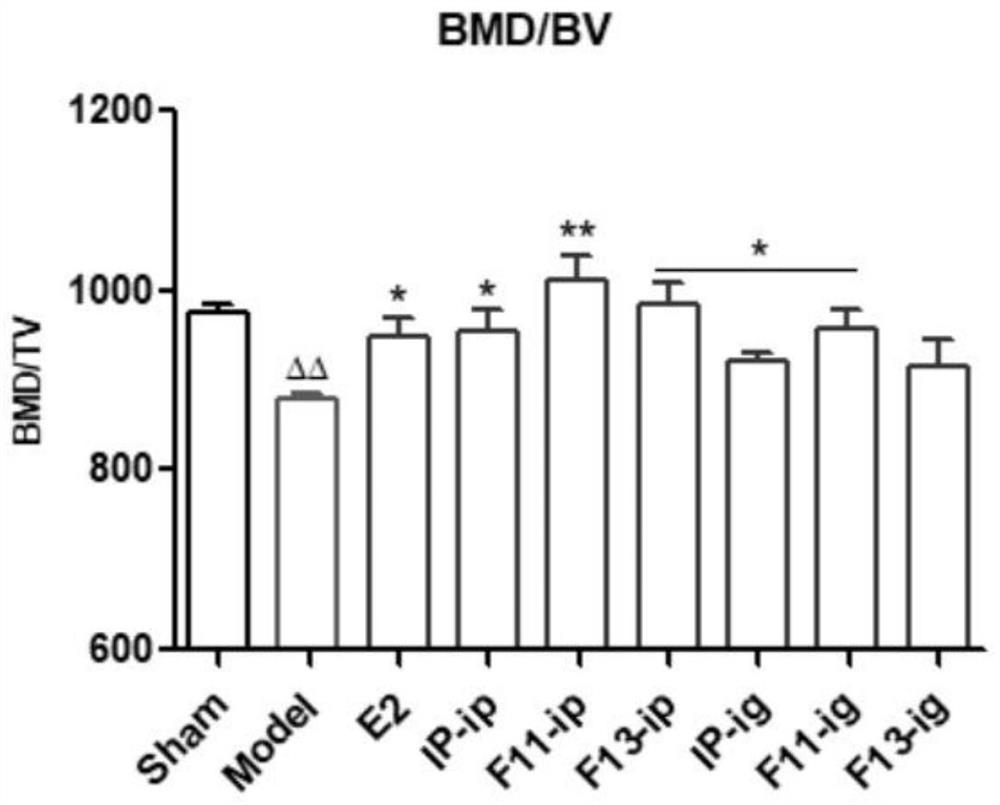
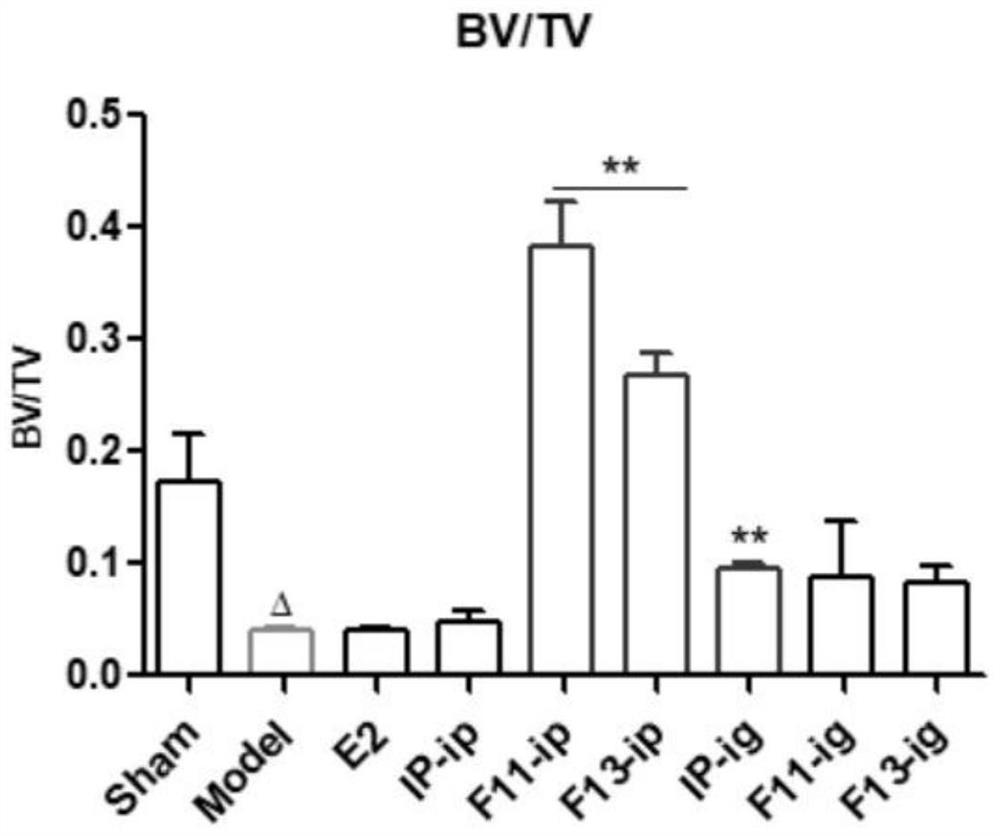
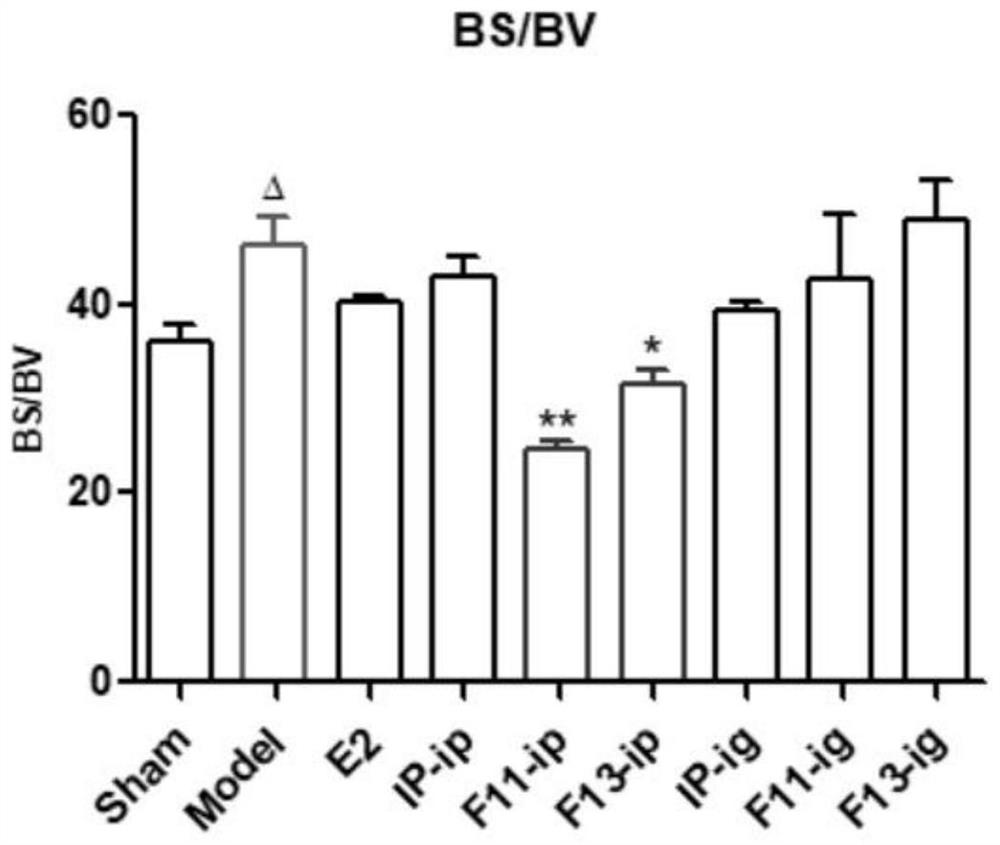
Application of formononetin derivative in preparation of medicine for treating or preventing perimenopausal syndrome
A formononetin, perimenopausal technology, applied in the field of drug synthesis to achieve the effects of increasing uterine weight, improving osteoporosis, and reducing perimenopausal syndrome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] In the present invention, the preparation method of the formononetin derivative preferably includes the following steps:
[0060] When the formononetin derivative has the structure shown in formula I, the preparation method comprises the following steps:
[0061] The compound having the structure shown in formula a, formaldehyde and the first amino compound are mixed and reacted to obtain the compound having the structure shown in formula I; the mole of the compound having the structure shown in formula a, formaldehyde and the first amine compound The ratio is 1:1:1; the structure of the first amine compound includes primary or secondary amino groups and R 3 group;
[0062]
[0063] R in said formula a 1 , R 2 The groups are the same as in formula I.
[0064] In the present invention, the compound having the structure shown in formula a is specifically preferably formononetin (structural formula shown in formula a-1) or daidin (structural formula shown in formula...
Embodiment 1
[0096] Embodiment 1: Preparation of F1
[0097] Dissolve 134.130mg (0.5mmol) of formononetin and 70mg (0.5mmol) of hexamethylenetetramine in 6mL of glacial acetic acid, stir at room temperature until completely dissolved, heat to reflux for 6h, keep at 100°C, and quickly add 2ml of 20% hydrochloric acid, stirred for 5min, cooled to room temperature, added 10mL of water, a brownish-yellow precipitate was obtained, and the brownish-yellow precipitate was collected to obtain a crude product, which was separated by silica gel column chromatography, and the reagents used were petroleum ether-ethyl acetate, petroleum ether and ethyl acetate The volume ratio of ester is 2:1, R f 0.11, the product F163.5 mg was obtained, and the yield was 47.31%.
[0098] The H NMR spectrum, carbon spectrum and mass spectrum data of the product are:
[0099] 1 H-NMR (400MHz, DMSO-D6) δ10.48(s, 1H), 8.48(s, 1H), 8.23(d, 1H), 7.52(dd, 2H), 7.12(dd, 2H), 7.01(d , 1H), 3.88(s, 3H).
[0100] 13 C-NMR...
Embodiment 2
[0103] Embodiment 2: the preparation of F2
[0104] Formononetin 67.065mg (0.25mmol) was completely dissolved in 1mL DMSO, and the prepared mixed solution [4mL methanol, 22.5μL 36% formaldehyde (0.25mmol) and 0.042mL pyrrolidine (0.25mmol)] was slowly added dropwise, Stir at room temperature for 2 hours, reflux for 10 hours, evaporate the solvent under reduced pressure to obtain a light red solid, collect the light red solid to obtain a crude product, and separate it by silica gel column chromatography. The reagents used are petroleum ether-methanol-ethyl acetate, petroleum ether, The volume ratio of methanol and ethyl acetate is 2:1:1, R f 0.108, the product F2 was obtained with a yield of 7.25%.
[0105] The H NMR spectrum, carbon spectrum and mass spectrum data of the product are:
[0106] 1 H-NMR (400MHz, DMSO-D6) δ8.35(s, 1H), 7.91(d, 1H), 7.49(d, 2H), 7.00(d, 2H), 6.85(d, 1H), 4.11(s , 3H), 3.78(s, 2H), 2.71(m, 4H), 1.80(m, 4H).
[0107] 13 C-NMR (400MHz, DMSO-D6) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com