Synthesis method of noradrenaline and bitartrate thereof
A norepinephrine and synthesis method technology, applied in the field of organic synthesis, can solve the problems of harsh reaction temperature and pH conditions, unsuitability for industrial production, cumbersome post-treatment process, etc., achieve low content, reduce recrystallization chiral resolution The effect of increasing the number of times and increasing the total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1, the preparation of norepinephrine of the present invention
[0066] 1. Preparation of intermediate III (compound A is R-(+)-N-benzyl-1-phenylethylamine)
[0067] Add 50.0g 3,4-dihydroxy-2'-chloroacetophenone, 113.5g R-(+)-N-benzyl-1-phenethylamine, 150ml N,N-dimethyl For formamide, heat up to 40-50°C to react, and monitor the reaction by TLC until the reaction of 3,4-dihydroxy-2'-chloroacetophenone is complete. The reaction solution was poured into 500ml of water, a large amount of solids were precipitated, filtered, stirred and washed with a mixed solution of 100ml ethyl acetate and 200ml petroleum ether, filtered and dried to obtain 90.1g of intermediate III with a molar yield of 92.9%.
[0068] 2. Preparation of intermediate IV (compound A is R-(+)-N-benzyl-1-phenylethylamine)
[0069] Add 80.0g of intermediate III, 400ml of tetrahydrofuran, and 400ml of ethanol to the reaction bottle in turn, stir until the solid is completely dissolved, add 25.4g of...
Embodiment 2
[0072] Embodiment 2, the preparation of norepinephrine of the present invention
[0073] 1. Preparation of intermediate III (compound A is R(+)-α-methylbenzylamine)
[0074] Add 10.0g 3,4-dihydroxy-2'-chloroacetophenone, 13.0g R(+)-α-methylbenzylamine, 40ml N,N-dimethylformamide to the reaction flask in sequence, heat up The reaction was carried out at 50-60°C, and the reaction was monitored by TLC until the conversion of 3,4-dihydroxy-2'-chloroacetophenone ceased. The reaction liquid was dropped into 120ml of water, a large amount of solids were precipitated, filtered, the filter cake was stirred and washed with a mixed solution of 20ml of ethyl acetate and 40ml of petroleum ether, filtered and dried to obtain 9.7g of intermediate III, with a molar yield of 66.7%.
[0075] 2. Preparation of intermediate IV (compound A is R(+)-α-methylbenzylamine)
[0076] Add 8.0g of intermediate III and 40ml of methanol successively in the reaction flask, stir until the solid is completely...
Embodiment 3
[0079] Embodiment 3, the preparation of norepinephrine bitartrate
[0080] Add the wet product of norepinephrine prepared in Example 1 or 2, 30.0g of water, 600ml of ethanol, and 24.8g of L-tartaric acid in sequence in the reaction flask, the solid is first dissolved and then precipitated, stirred evenly, and filtered to obtain a salt-forming solid (wet product).
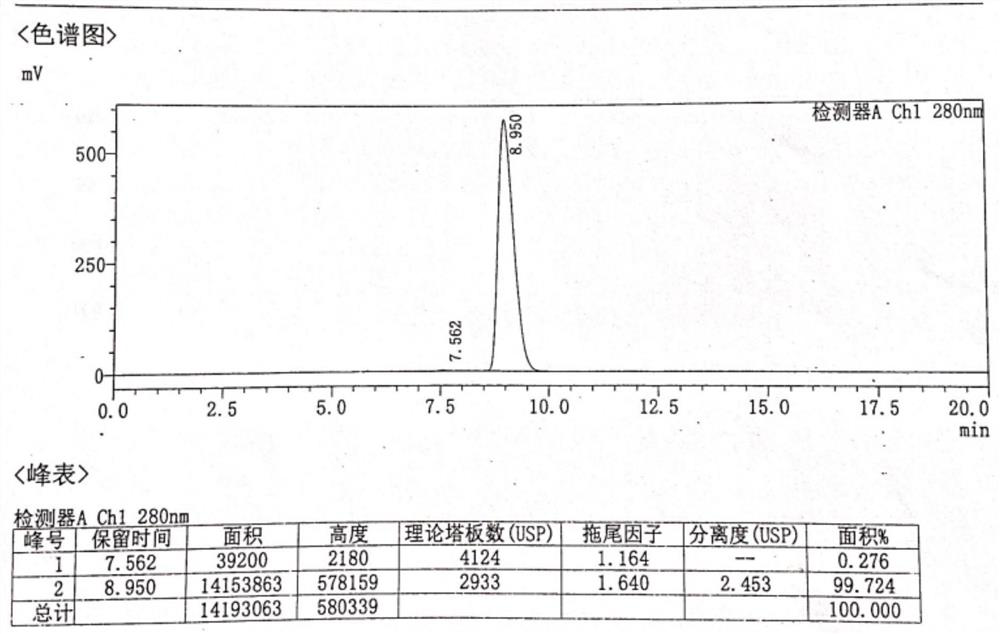
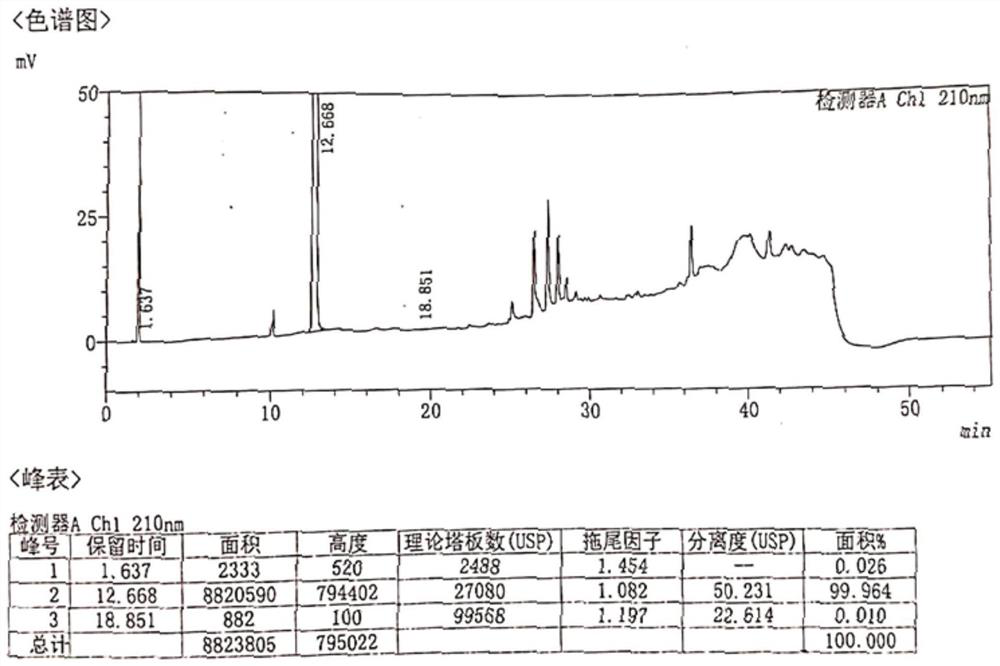
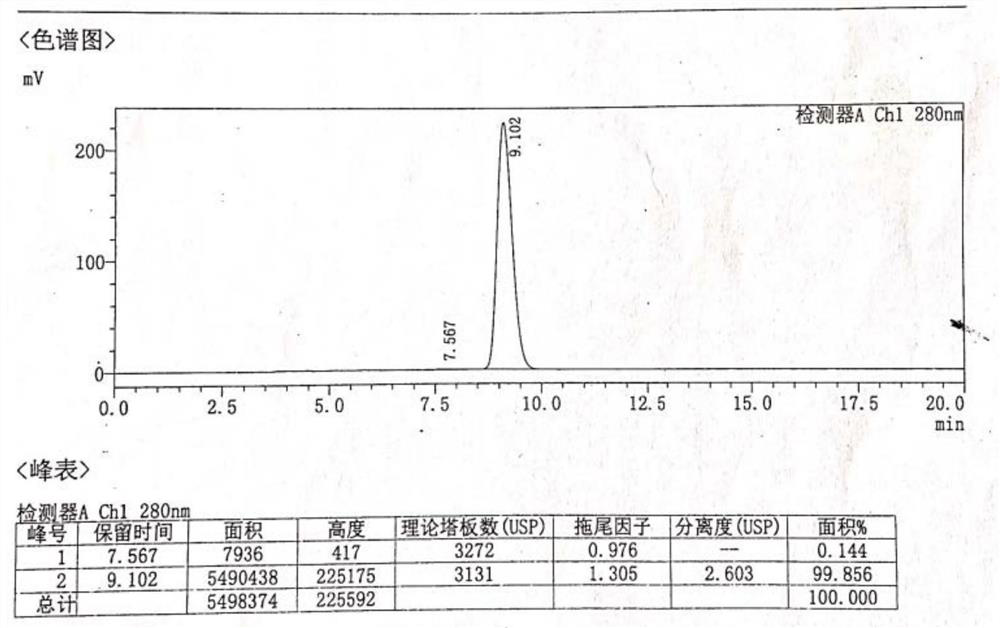
[0081] The resulting solid was added to a reaction flask, 30.0 g of water and 800 ml of ethanol were added, heated until the solid was completely dissolved, turned off the heating, crystallized naturally, filtered, and dried to obtain 17.7 g of norepinephrine bitartrate (Compound I), the total molar yield 31.8% (based on intermediate V). HPLC purity 99.964%, isomer content 0.144%. HPLC purity chart see figure 2 , isomer content map see image 3 . It shows that making bitartrate further increases the content of norepinephrine in R-configuration. After only one chiral resolution of crystallization, the norepine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com