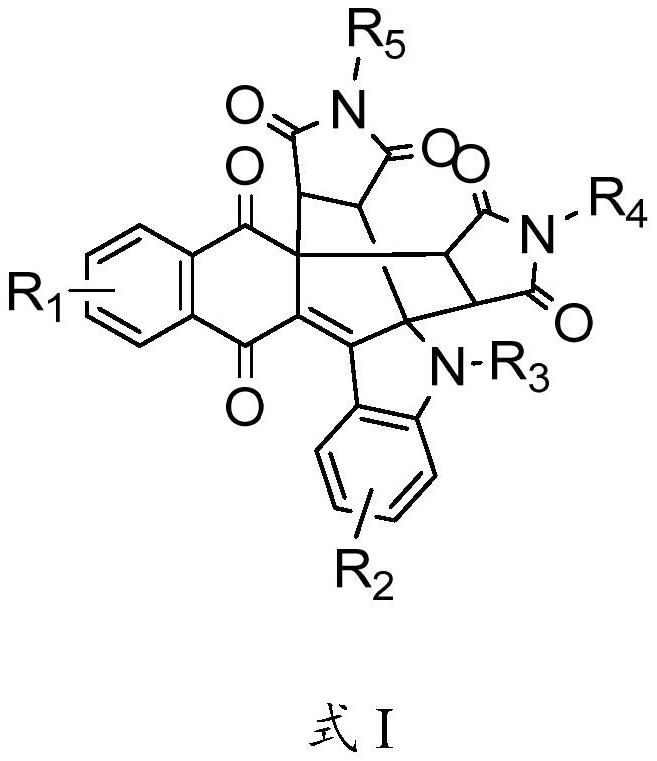
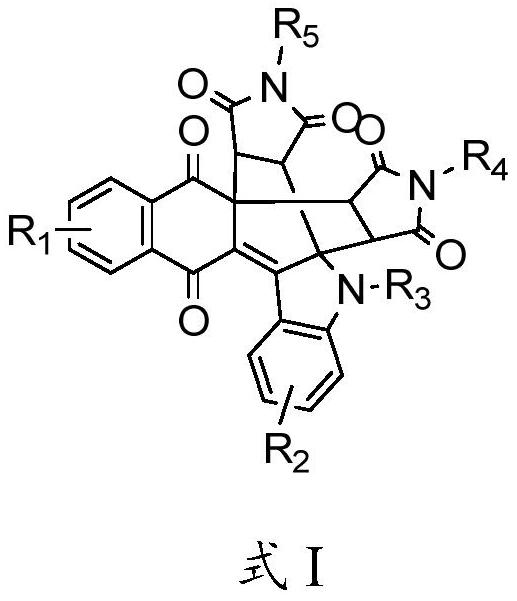
Naphthoquinone multi-fused-ring derivative as well as preparation method and application thereof
A derivative, naphthoquinone technology, applied in the field of medicinal chemistry, can solve the problems of harsh reaction conditions, pre-functionalization, etc., and achieve the effects of simple operation, low cost, and wide substrate adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
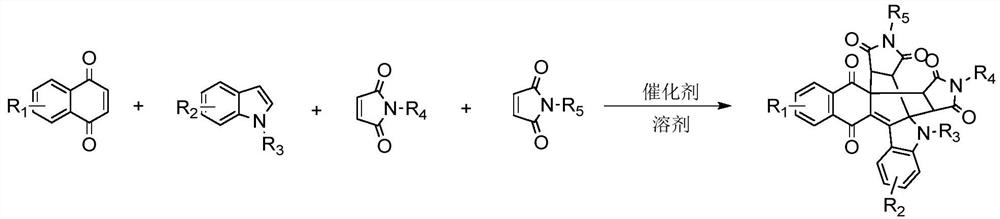
[0036] Second, the invention provides a method for preparing naphthoquinone polycyclic derivatives, comprising the steps of:
[0037] Naphthoquinone, indole and maleimide are put into a reaction vessel, and then a catalyst and a solvent are added, and the naphthoquinone polycyclic derivatives are obtained through reaction, separation and purification.
[0038] In the present invention, the chemical reaction formula is,
[0039]
[0040] In the present invention, the molar ratio of naphthoquinone, indole and maleimide is 1:0.5-3:0.5-4.
[0041] In the present invention, the molar ratio of naphthoquinone to catalyst is 1:0.01-0.5.
[0042] In the present invention, the catalyst includes TsOH·H 2 O, CuSO 4 ·5H 2 O, Cu(OTf) 2 , Ni(OTf) 2 , Pd(OAc) 2 、Fe(acac) 3 , FeCl 3 , HCl, B(C 5 f 6 ) 3 、CoCl 2 、Ni(acac) 2 、C 6 h 5 B(OH) 2 , BF 3 ·OEt 2 at least one of the
[0043] In the present invention, the solvent includes EtOH, H 2 O, THF, toluene, (CH 2 Oh) 2 ,...
Embodiment 1
[0048] Synthesis of embodiment 1 naphthoquinone polycyclic derivative 4a
[0049]
[0050]Add 47.4mg 1,4-naphthoquinone, 39.3mg N-methylindole, 52mg N-phenylmaleimide, 7.7mg tris(pentafluorophenyl)borane, and then add 3mL acetonitrile to the reaction flask , reacted at 100°C for 24h, cooled to room temperature, added saturated saline, extracted with dichloromethane, combined the organic phases, extracted with anhydrous magnesium sulfate, and obtained red solid 4a by column chromatography with a yield of 80%.
[0051] Red solid, melting point: 282-283°C. 1 H NMR (400MHz, DMSO-d 6 ):δ9.14(d, J=8.0Hz,1H),8.21–8.15(m,2H),7.88–7.81(m,2H),7.47(ddd,J=8.3,7.2,1.2Hz,1H), 7.33–7.22(m,6H),6.89(d,J=8.4Hz,1H),6.75(t,J=7.6Hz,1H),6.65–6.57(m,4H),4.28(d,J=8.5Hz ,2H),4.05(d,J=8.5 Hz,2H),3.42(s,3H). 13 C NMR (101MHz, DMSO-d 6 ): δ194.1, 178.5, 174.7, 172.0, 159.0, 153.5, 137.3, 136.0, 135.4, 135.2, 134.1, 131.7, 129.7, 129.5, 129.1, 127.8, 126.7, 126.6, 118.5, 1167.4, 2, 191 46.0, 41....
Embodiment 2
[0052] The synthesis of embodiment 2 naphthoquinone polycyclic derivatives 4b
[0053]
[0054] Add 47.4mg of 1,4-naphthoquinone, 35.1mg of indole, 52mg of N-phenylmaleimide, 15.4mg of tris(pentafluorophenyl)borane to the reaction bottle, then add 3mL of acetonitrile, and react at 120°C After 24 hours, after cooling to room temperature, saturated saline was added, and then extracted with dichloromethane. The organic phases were combined, extracted with anhydrous magnesium sulfate, and column chromatography gave a red solid 4b with a yield of 54%.
[0055] Orange solid, melting point: 208-210°C. 1 H NMR (400MHz, CDCl 3 ):δ9.22(d, J=8.2Hz, 1H), 8.37–8.32(m, 1H), 8.29–8.24(m, 1H), 7.77–7.71(m, 2H), 7.46(t, J=7.7 Hz, 1H), 7.23(dd, J=6.8, 4.4Hz, 6H), 7.01(d, J=8.2Hz, 1H), 6.95(t, J=7.7Hz, 1H), 6.70(dd, J=6.7 , 2.9Hz, 4H), 5.93(s, 1H), 4.14(d, J=8.7Hz, 2H), 3.58(d, J=8.7Hz, 2H).13C NMR (101MHz, CDCl 3 ): δ 193.9, 179.0, 173.3, 171.1, 158.6, 153.3, 136.9, 135.8, 135.0, 134.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com