Reaction catalyst for preparing gamma-butyrolactone through maleic anhydride hydrogenation, and preparation method and application thereof
A technology of maleic anhydride and catalyst is applied in the reaction catalyst for producing γ-butyrolactone by hydrogenation of maleic anhydride and its preparation and application fields, and achieves low cost, high target product selectivity and excellent hydrogenation activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
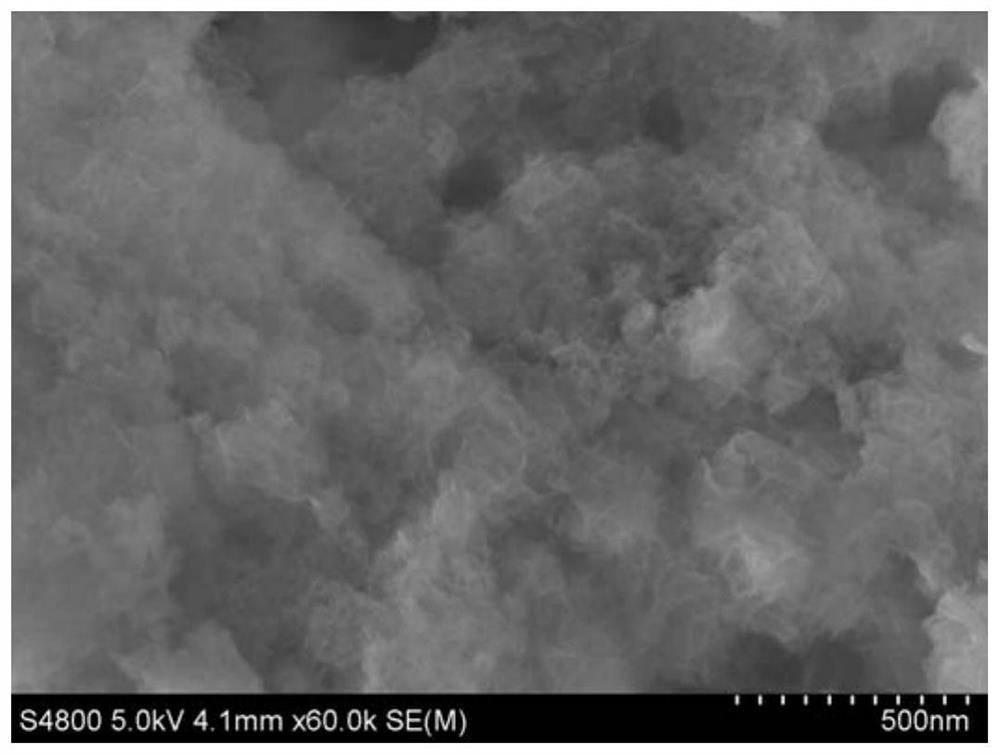
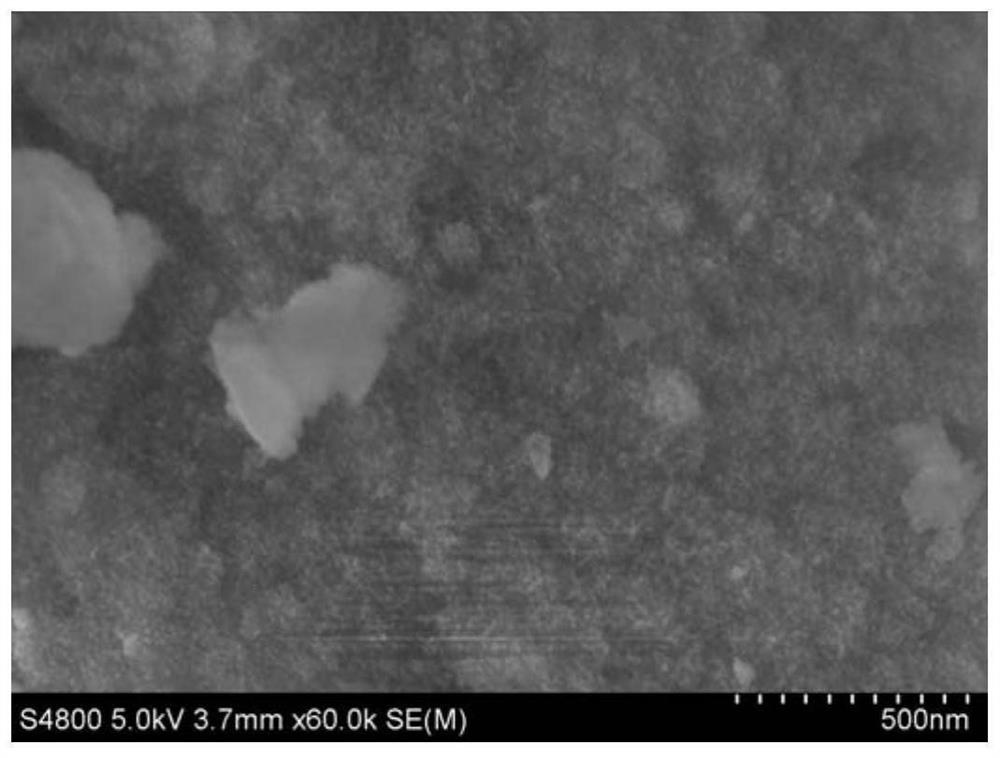
Image
Examples
Embodiment 1
[0041] One, the preparation of 40wt%Ni-MFI catalyst
[0042] (1) In the mixed solution of ethyl orthosilicate and water, add a certain amount of tetrabutylammonium hydroxide aqueous solution dropwise, stir for 3h to obtain a molar composition of 1.0 ethyl orthosilicate: 3.0 tetrapropylammonium hydroxide: 56 solution in water;
[0043] (2) Put the obtained transparent solution into a stainless steel hydrothermal kettle, and crystallize at 170°C for 4 days;
[0044] (3) The obtained white powder was centrifuged, dried at 100° C. for 10 hours, and calcined at 550° C. for 6 hours to obtain an MFI molecular sieve with a microporous structure.
[0045] (4) Dissolve nickel nitrate hexahydrate in water, add a concentration of 25% ammonia, stir for 10 minutes and stir with the above-mentioned microporous structure MFI molecular sieve for 5 hours, the mass composition of the suspension is 1.0 carrier: 0.67 metal theoretical load mass: 15 Ammonia water: 200% water, then distill ammonia...
Embodiment 2
[0060] The difference between Example 2 and Example 1 is that the prepared catalyst is a catalyst 40wt% Ni-PS using silica sol as a silicon source, wherein 40wt% alkaline silica sol is added to the nickel-ammonia complex solution.
[0061] The specific method for the preparation of 40wt%Ni-PS is as follows: Ni(NO 3 ) 2 6H 2 Dissolve O in water, add 25% ammonia and stir for 100 minutes to obtain a nickel-ammonia complex solution, then add alkaline silica sol drop by drop, the mass ratio of each component of the suspension is: 1.0 carrier: 0.67 metal theoretical load Quality: 15% ammonia water (25wt%): 200% water, stir for 5 hours; then distill ammonia at 80°C for 8h, put the suspension in a non-closed container, use the volatilization characteristics of ammonia at 80°C to make the ammonia in the suspension The content gradually decreased until the pH=7, then washed, filtered and dried, and after roasting at 550°C for 5h, a 40wt% Ni-PS catalyst oxide precursor was obtained; t...
Embodiment 3
[0065] The difference between Example 3 and Example 1 is that the prepared catalyst uses mesoporous silicon (ie commercial silica carrier) as the carrier of 40 wt% Ni-MSI catalyst, and mesoporous silicon powder is added to the nickel ammonium complex solution.
[0066] The specific method of the preparation of 40wt%Ni-MSI is as follows: Ni(NO 3 ) 2 6H 2 O was dissolved in water, stirred for 15 min after adding ammonia water to obtain a nickel-ammonia complex solution, then added mesoporous silicon powder in batches, the mass ratio of each component of the suspension was: 1.0 carrier: 0.67 metal theoretical load mass: 15 ammonia water ( 25wt%): 200% water, stirred for 5h; then distilled ammonia at 80°C for 8h until pH = 7, washed, filtered and dried, and roasted at 550°C for 5h to obtain a 40wt% Ni-MSI catalyst oxide precursor; 2 Reduction at 500°C for 2 hours under hydrogen atmosphere, hydrogen space velocity is 2000h -1 , to obtain the target catalyst 40wt% Ni-MSI.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com