Isothermal nucleic acid amplification sensor for rapidly detecting hypochlorous acid and myeloperoxidase
An isothermal nucleic acid amplification and sensor technology, applied in the field of nanomaterial synthesis and molecular detection, can solve the problems of unfavorable long-term storage, background fluorescence, difficult quantitative measurement, etc., to prevent false positive signals, improve detection efficiency, and stabilize long-term storage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: synthetic probe P1, P2 and Lock-In
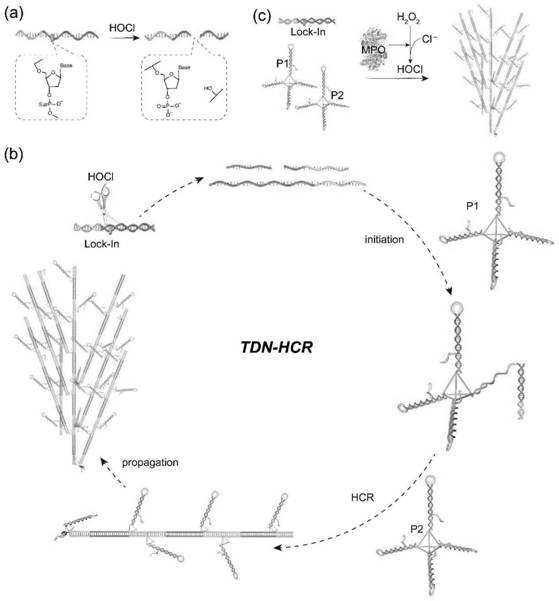
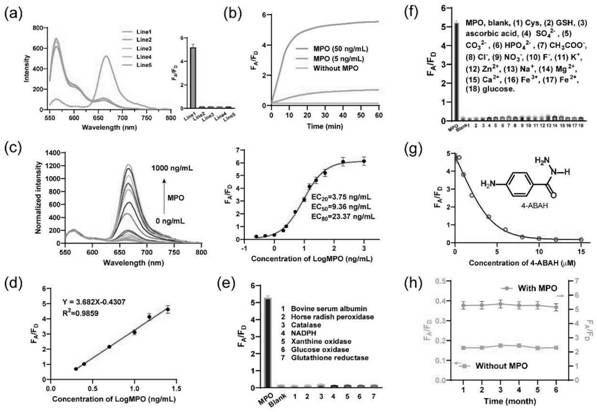
[0054] The nucleic acid nanosensor of the present invention comprises three parts: a, probe 1 (P1), respectively constitute tetrahedral rigid nanostructure by five kinds of DNA single strands (S1, S2, S3, S4 and H1); b, probe 2 (P2), respectively composed of five DNA single strands (S1, S2, S3, S4 and H2) to form a tetrahedral rigid nanostructure, wherein H1 and H2 constitute two enzyme-free amplification reactions such as hybridization chain reaction (HCR). Unit; c, probe 3 (Lock-In), phosphorothioate-modified nucleic acid constitutes a lock chain (Lock), which can initiate a chain reaction of hybridization (In), wherein In is initially locked by Lock and cannot initiate HCR, When target myeloperoxidase is present, it catalyzes H 2 o 2 And chloride ions (Cl-) to produce hypochlorous acid (HOCl), the phosphorothioate chain breaks, and then releases In, which triggers H1 and H2 to undergo enzyme-free amplification reac...
Embodiment 2
[0059] Embodiment 2: Electrophoretic characterization of probes
[0060] (1) HOCl cleavage of Lock-In: fluorescently labeled Lock (2 μM) or fluorescently labeled Lock-In (2 μM) was incubated with different concentrations of hypochlorous acid in 1×PBS buffer at 37° C. for 1 h. The Lock-In and cleavage products were characterized by 10% polyacrylamide gel electrophoresis. Under the conditions of 4°C and 120V, electrophoresis separation was carried out for 80 minutes. Place the gel into the GelImage system at room temperature for imaging observation.
[0061] (2) HOCl triggers classical HCR: 2 μM each of h1, h2, In or Lock-In was incubated with different concentrations of hypochlorous acid in 1×PBS buffer at 37° C. for 4 h. Use 10% polyacrylamide gel electrophoresis to characterize chains and cleavage products. Under the conditions of 4°C and 120V, electrophoresis separation was carried out for 80 minutes. After staining the gel in GelRed for 15 min at room temperature, put t...
Embodiment 3
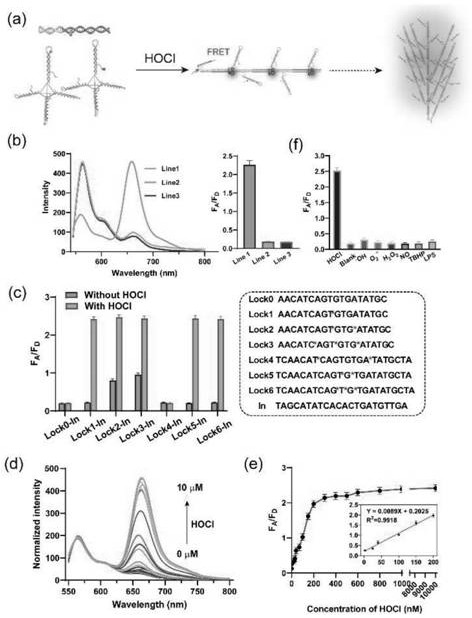
[0064] Embodiment 3: Probe detects hypochlorous acid in vitro
[0065] (1) Synthetic probes
[0066] (1) Synthesis of fluorescently labeled probe P1: Mix 1 μM of S1, S2, S3 and S4 with 4 μM H1 (Cy3 labeled) in Tris-HCl-Mg 2+ Buffer (20mM Tris-HCl, 50mM MgCl 2 , pH=8.0), mixed and centrifuged, heated at 95°C for 5min, immediately cooled on ice cubes, and stored at 4°C until use. The obtained probes were used directly in subsequent experiments without further isolation or purification.
[0067] (2) Synthesis of fluorescently labeled probe P2: Mix 1 μM of S1, S2, S3 and S4 with 4 μM H2 (Cy5 labeled) in Tris-HCl-Mg 2+ Buffer (20mM Tris-HCl, 50mM MgCl 2 , pH=8.0), mixed and centrifuged, heated at 95°C for 5min, immediately cooled on ice cubes, and stored at 4°C until use. The obtained probes were used directly in subsequent experiments without further isolation or purification.
[0068] (2) In vitro identification and results
[0069] Fluorescence spectrum in response to hyp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com