Anti-drug-resistance antibacterial amidine oligomer as well as preparation method and application thereof
A production method and oligomerization-like technology, applied in antibacterial drugs, resistance to vector-borne diseases, organic chemistry, etc., can solve problems such as high toxicity, and achieve the effect of low toxicity, high therapeutic index, and broad-spectrum antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] After testing, a kind of antibacterial amidine oligomer with broad-spectrum antibacterial property, its chemical formula is as follows:
[0032]
[0033] Wherein, 5≤n≤8, R1 and R2 have the structure in the above description. It can be seen from the above structural formula that 81 compounds can be obtained through different combinations of R1 and R2. In order to facilitate the analysis and description of these compounds, 60 compounds are labeled in Table 1 below. It should be noted that, For those skilled in the art, unlabeled compounds can also be synthesized, and the properties and synthesis methods of these unlabeled compounds are similar to those of the 60 labeled compounds, so no further analysis and description are made in the present invention.
[0034] The structural formulas of the 60 compounds and their antibacterial activities and biocompatibility lists are shown in Table 1 and Table 2. It can be seen from Table 2 that, compared with other compounds, the t...
Embodiment 2
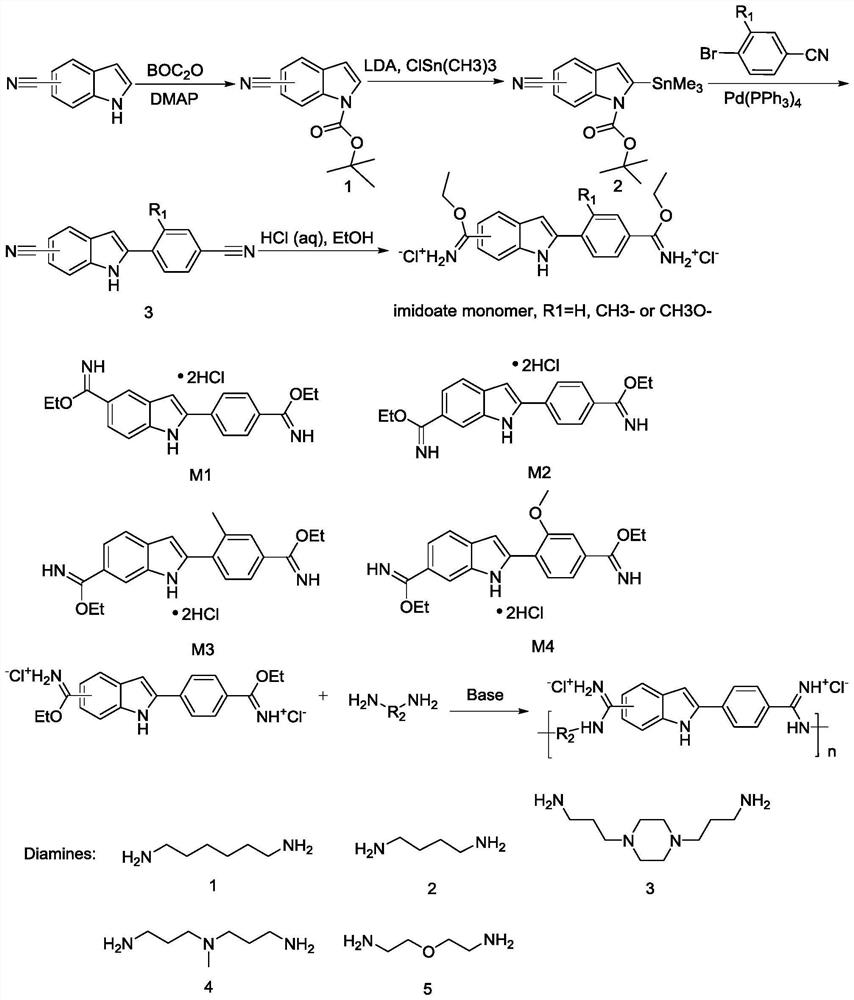
[0041] As shown in Figure 1, the present invention also discloses a method for synthesizing an antibacterial amidine oligomer with drug resistance, comprising the steps of:
[0042] S1: prepare a compound monomer with the following molecular formula:
[0043]
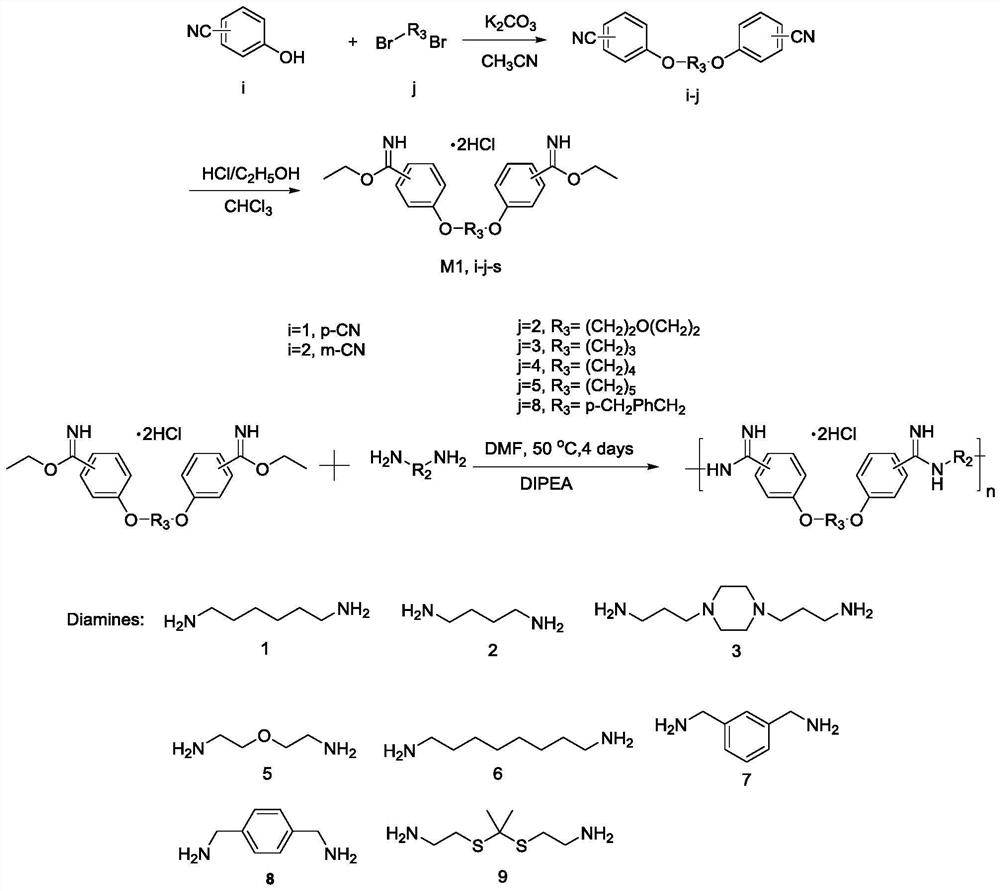
[0044] S2: react the compound monomer with H2N-R2-NH2, anhydrous N,N-dimethylformamide (DMF) and N,N-diisopropylethylamine (DIPEA) to obtain the amidines The oligomers, R1 and R2 have the structures described above.
[0045] Wherein, the above step S1 includes the following steps:
[0046] S11: Weigh 5-cyanindole or 6-cyanindole and di-tert-butyl dicarbonate, then add dichloromethane and mix evenly, add a catalytic amount of 4-dimethylaminopyridine (DMAP) at 2-6 After stirring at ℃ for half an hour, return to room temperature of 20-25℃ and stir overnight, filter and wash the mixture and spin dry with a rotary evaporator to obtain a solid product, and then purify the product with a silica gel column to obtain a whit...
experiment example 1
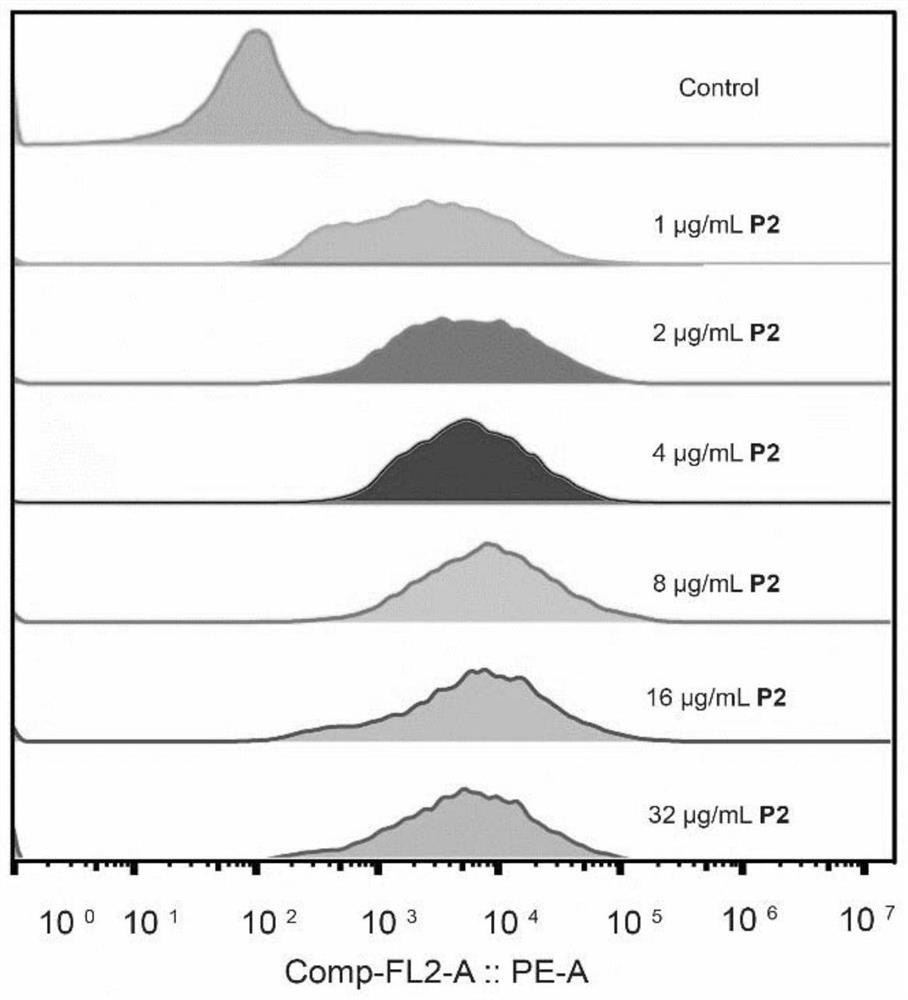
[0053] This experimental example is described in detail with the P2 compound:
[0054] Materials and methods: All reagents were provided by TCI (USA), Sigma-Aldrich, McLean, Adamas and other organic reagent companies and Beyontian Biotechnology Co., Ltd., and were used directly without further purification (unless otherwise specified). The ultrapure water used in the experiment came from a Milli-Q purification instrument. The inert gas is nitrogen.
[0055] Synthesis of P2 The synthesis steps are shown in Figure 1. Carry on the synthesis of 4',6-diimido-2-phenylindole hydrochloride monomer firstly. Weigh 6-cyanindole (10g, 70.3mmol) and di-tert-butyl dicarbonate (BOC) (35.36g, 162mmol), then add 100mL of dichloromethane and mix well, then add a catalytic amount of 4-dimethylamino Pyridine (DMAP) (0.86g, 7.03mmol), stirred at room temperature for 12-24h to obtain a mixture, the mixture was extracted and spin-dried to obtain a solid mixture, and the solid mixture was separate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com