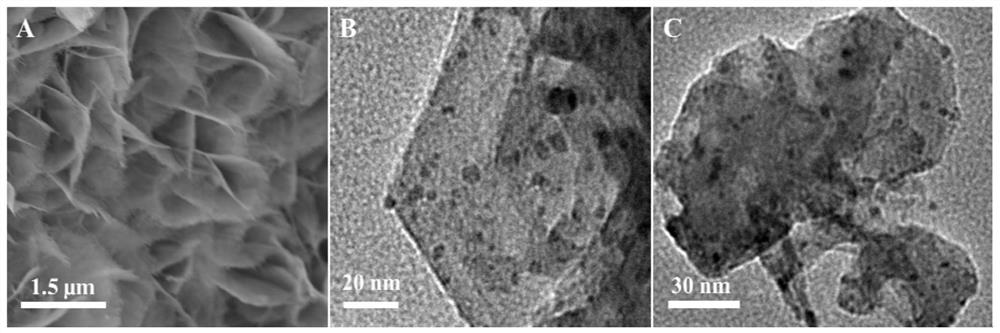
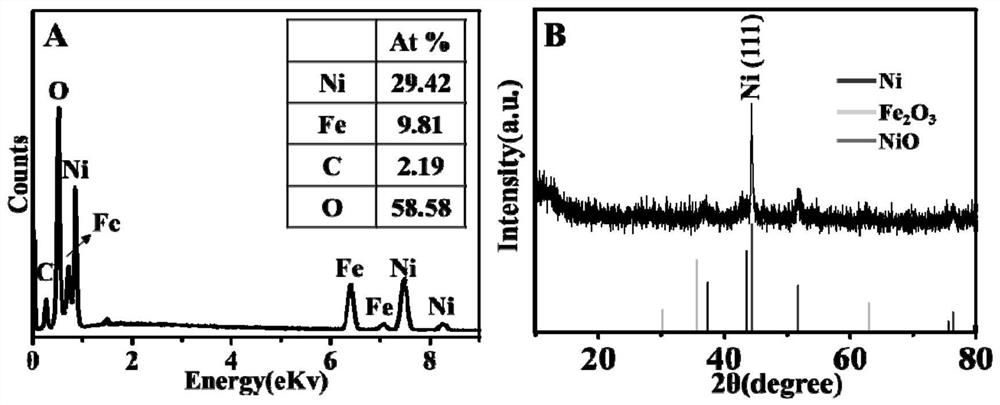
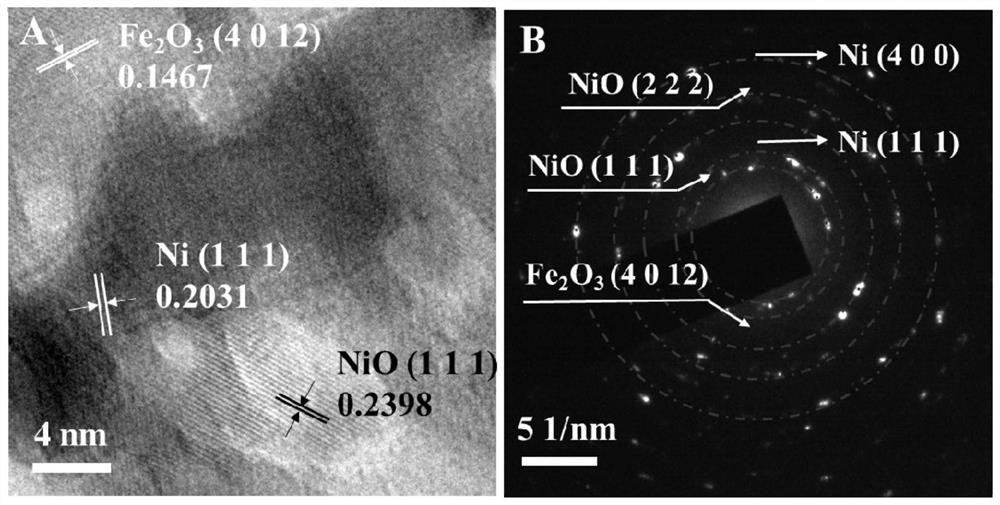
Preparation of layered nickel/ferronickel double-metal oxide nano composite material
A technology of double metal oxides and nanocomposites, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve problems such as low hydrogen evolution overpotential, environmental pollution, and consumption of fossil energy, and achieve Increased specific surface area, high purity, safe and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Step 1: Weigh 0.1g NiCl 2 ·6H 2 O, 0.06g Fe(NO 3 ) 3 9H 2 Dissolve O in 60mL of deionized water, stir to dissolve evenly, then add 0.27g of urea, stir to dissolve, cover and seal, place in an electric heating constant temperature blast drying oven, and raise the temperature from room temperature to 140°C at a rate of 5°C / min. And keep it warm at 140°C for 8h. After cooling down to room temperature naturally, the precipitate was extracted by centrifugation, washed alternately with deionized water and absolute ethanol, and then dried in a drying oven for 12 hours.
[0065] The second step: Pour the obtained product into a magnetic boat, spread it and transfer it to a tube furnace in a nitrogen atmosphere, raise the temperature from room temperature to 400°C at a rate of 10°C / min, and heat at a constant temperature for 4h. Then cooled to room temperature, taken out and sealed for storage in subsequent experiments.
[0066] The third step: Pour the resulting product i...
Embodiment 2
[0072] Step 1: Weigh 0.06g NiCl 2 ·6H 2 O, 0.05g Fe(NO 3 ) 3 9H 2 Dissolve O in 60mL of deionized water, stir to dissolve evenly, then add 0.6g of urea, stir to dissolve, cover and seal, place in an electric heating constant temperature blast drying oven, and raise the temperature from room temperature to 120°C at a rate of 5°C / min. And keep it warm at 120°C for 10h. After cooling down to room temperature naturally, the precipitate was extracted by centrifugation, washed alternately with deionized water and absolute ethanol, and then dried in a drying oven for 12 hours.
[0073] The second step: Pour the obtained product into a magnetic boat, spread it and transfer it to a tube furnace in a nitrogen atmosphere, raise the temperature from room temperature to 400°C at a rate of 10°C / min, and heat at a constant temperature for 4h. Then cooled to room temperature, taken out and sealed for storage in subsequent experiments.
[0074] The third step: Pour the resulting product ...
Embodiment 3
[0078] Step 1: Weigh 0.05g NiCl 2 ·6H 2 O, 0.08g Fe(NO 3 ) 3 9H 2 O was dissolved in 60mL of deionized water, stirred and dissolved evenly, then 0.27g of urea was added, stirred and dissolved, sealed, placed in an electric heating constant temperature blast drying oven, and the temperature was raised from room temperature to 160°C at a rate of 5°C / min. And keep it warm at 160°C for 12h. After cooling down to room temperature naturally, the precipitate was extracted by centrifugation, washed alternately with deionized water and absolute ethanol, and then dried in a drying oven for 12 hours.
[0079] Step 2: Pour the obtained product into a magnetic boat, lay it flat and transfer it to a tube furnace in a nitrogen atmosphere, raise the temperature from room temperature to 400°C at a rate of 10°C / min, and heat at a constant temperature for 3h. Then cooled to room temperature, taken out and sealed for storage in subsequent experiments.
[0080] The third step: Pour the resul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com