A kind of silfluorenyl conjugated porous polymer and preparation method thereof
A porous polymer and silfluorenyl technology, applied in the field of silfluorenyl conjugated porous polymer and its preparation, can solve the problems of poor light stability, photobleaching, etc., achieve short synthesis route, simple preparation method, and raw material preparation technology mature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
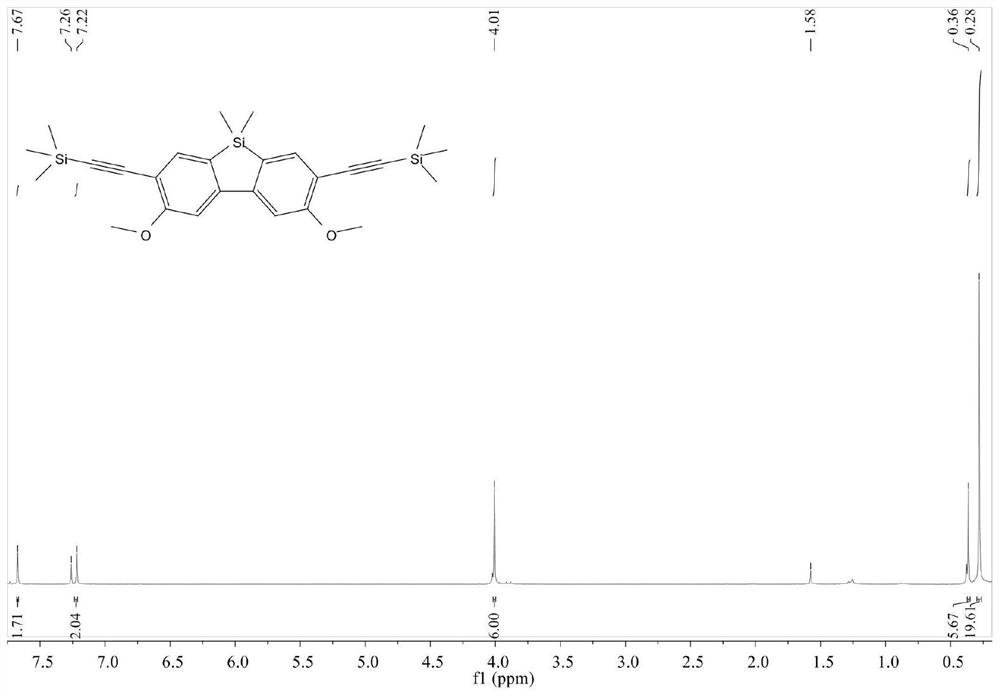
[0038] This example specifically introduces the synthesis process of the silicon compound B (3,6-dimethoxy-2,7-diethynyl-9,9-dimethylsilylfluorene).
[0039] Step 1: In an argon atmosphere, using 2,7-dibromo-3,6-dimethoxy-9,9-dimethylsilylfluorene and trimethylsilyne as raw materials, through Sonogashira coupling reaction to obtain 2,7-Ditrimethylsilyne-3,6-dimethoxy-9,9-dimethylsilylfluorene, of which 2,7-dibromo-3,6-dimethoxy-9,9 - The molar ratio of dimethylsilylfluorene and trimethylsilyne is 1:3.
[0040]The specific operation is: weigh 3,3'-dibromo-4,4'-dimethoxybiphenyl (3.72g, 10mmol), cuprous iodide (0.20g, 1mmol), tetrakistriphenylphosphine palladium ( 2.32 g, 2 mmol) in the reaction device, and under stirring, the argon was replaced by vacuum three times. Trimethylsilyne (2.94 g, 30 mmol) was added dropwise to a glass bottle placed on a balance, 50 mL of distilled piperidine was added, deoxygenated for 15 minutes, and then transferred to an argon-filled reaction d...
Embodiment 2
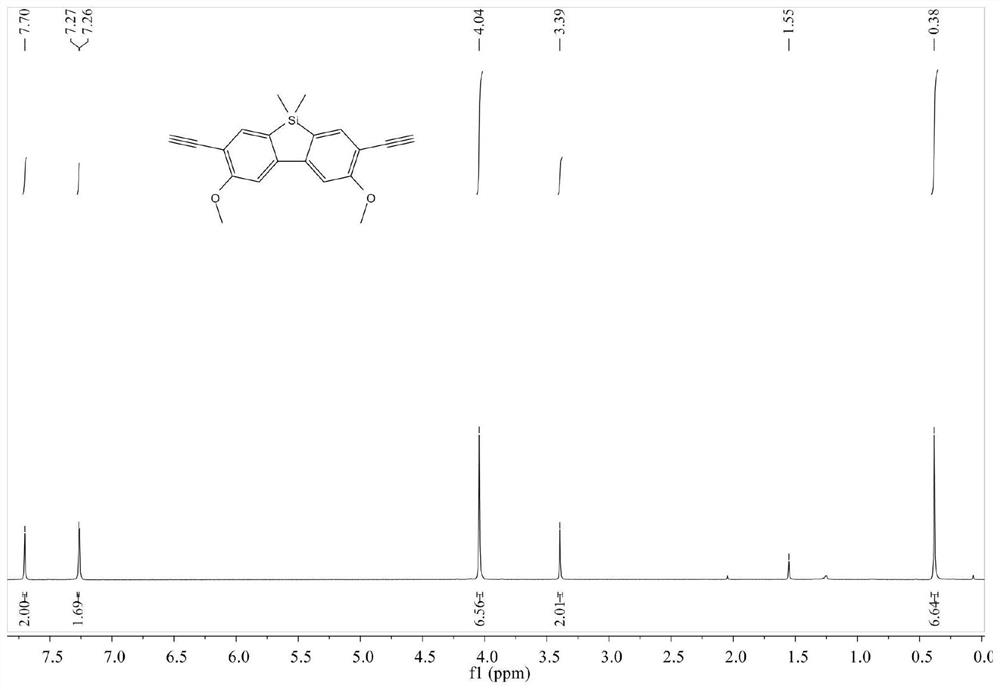
[0055] This example specifically describes the synthesis process of the silicofluorene-based conjugated porous polymer with light-induced fluorescence enhancement effect.
[0056] Step S1, copolymerization process: first take by weighing B (50mg, 0.11mmol), cuprous iodide (3mg, 0.02mmol), tetrakistriphenylphosphine palladium (34mg, 0.03mmol) and put it into the reaction device (two-necked bottle, loaded There is a reflux tube, with a tee on the reflux tube), and the argon is replaced by vacuum three times under stirring; then 1,3,5-triiodobenzene (46mg, 0.10mmol), mixed solvent (tetrahydrofuran: triethylamine=1: 7, 10 mL), heated to 80 °C, solids were precipitated and attached to the bottle wall within 1 hour of the reaction, and the reaction was continued for 3 days. Saturated aqueous ammonium chloride solution was added at room temperature to quench, and suction filtered to obtain a pale yellow solid, that is, the crude product.
[0057] Step S2, impurity removal process: t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com