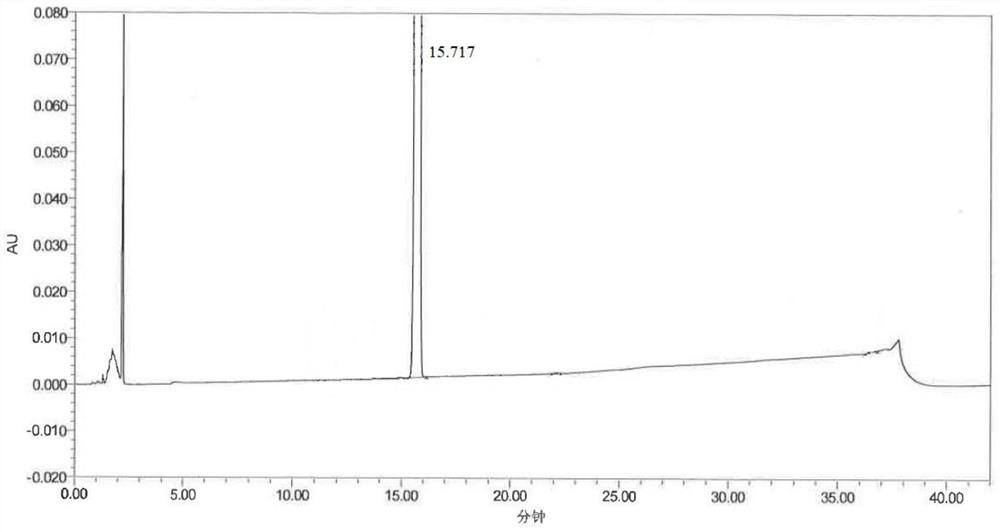
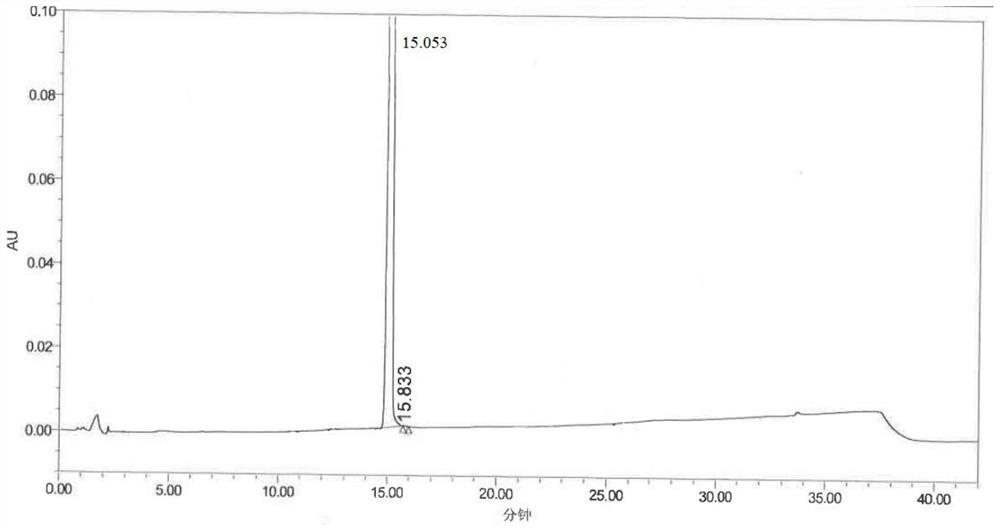
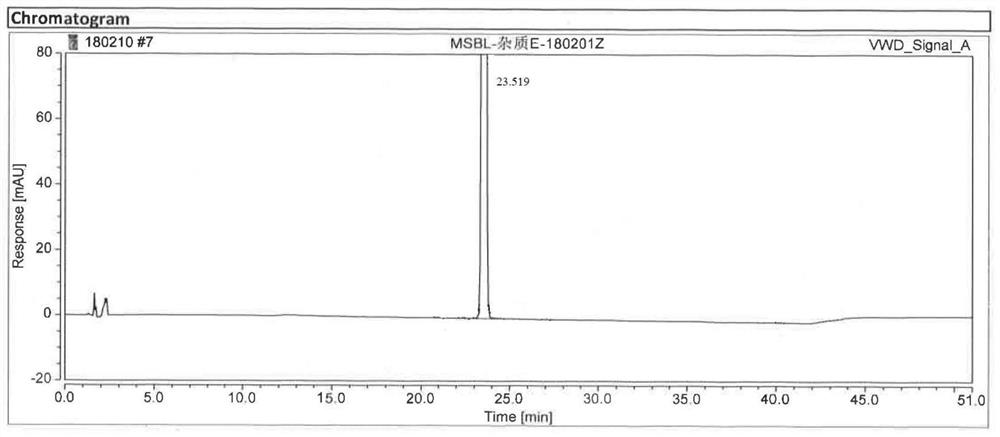
Mosapride impurity compound
A technique for mosapride citrate and compounds, which is applied in the field of drug synthesis, can solve the problems affecting the quality of mosapride citrate raw materials and difficult purification, and achieve short routes, high product purity and high reaction yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add ethyl 2-ethoxy-4-acetamidobenzoate (25.13g, 0.1mol) and NBS (23.14g, 0.13mol) into DMF (120mL), heat at 70°C for 3h, and detect the reaction Pour the reaction solution into ice water and stir to crystallize, filter, wash the filter cake with water (50ml×2), and dry to obtain ethyl 2-ethoxy-4-acetamido-5-bromobenzoate as a white solid with a yield of 98.7% , with a purity of 99.95%.
Embodiment 2
[0045] Add ethyl 2-ethoxy-4-acetamidobenzoate (25.13g, 0.1mol) and NBS (17.80g, 0.1mol) into methanol (120mL), heat at 50°C for 3h, and detect the reaction Pour the reaction solution into ice water and stir to crystallize, filter, wash the filter cake with water (50ml×2), and dry to obtain ethyl 2-ethoxy-4-acetamido-5-bromobenzoate as a white solid with a yield of 94.2% , with a purity of 99.85%.
Embodiment 3
[0047] Add ethyl 2-ethoxy-4-acetamidobenzoate (25.13g, 0.1mol) and NBS (32.04g, 0.18mol) into acetonitrile (120mL), heat at 80°C for 3h, and detect the reaction Pour the reaction solution into ice water and stir to crystallize, filter, wash the filter cake with water (50ml×2), and dry to obtain ethyl 2-ethoxy-4-acetamido-5-bromobenzoate as a white solid with a yield of 93.4% , with a purity of 99.76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com