Mitochondria-targeted peroxynitrite/bisulfite double-response fluorescent probe
A technology of peroxynitrite and bisulfite, applied in fluorescence/phosphorescence, material analysis by optical means, luminescent materials, etc., can solve the problem of inability to detect two molecules at the same time, lack of biological targeting of probes, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Mitochondrial targeted peroxy-nitrate / sulfite dual response fluorescent probe
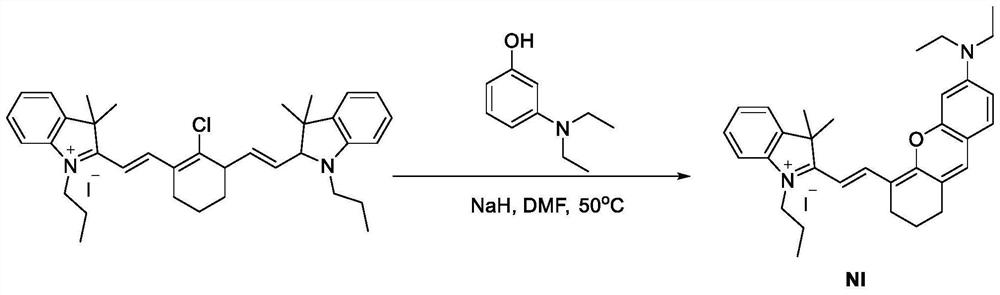
[0034] like figure 1 The synthesis road map shows that the compound Ni synthesis: Under nitrogen protection, aminophenols (5 g, 30 mmol), NaH (0.73 g, 30 mmol) were dissolved in 50 ml DMF, and stirred at room temperature for 10 min to give a reaction Liquid; then IR-780 (10.0 g, 15 mmol) was dissolved in 100 ml DMF to give a secondary reaction solution, and the solution was slowly added dropwise to the primary reaction solution, 50 ° C for 5 hours. The TLC showed completely, the solvent was dried, and the crude product was separated from the column chromatography (dichloromethane: methanol = 100: 1 to 10: 1) to give 3.9 g of red solid.
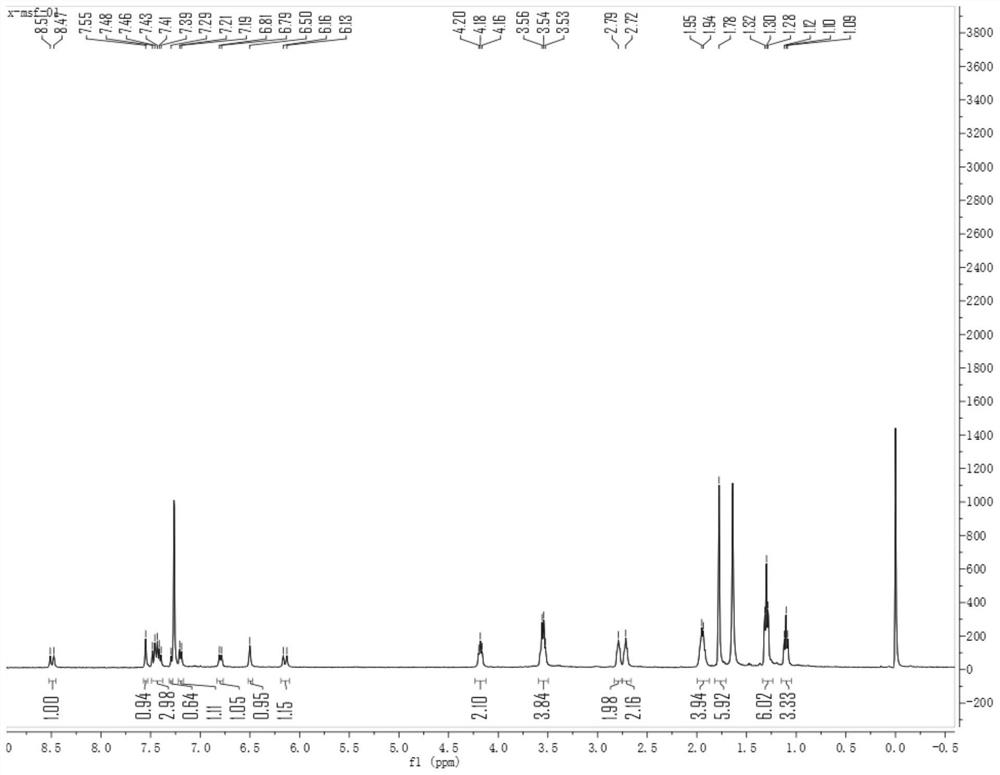
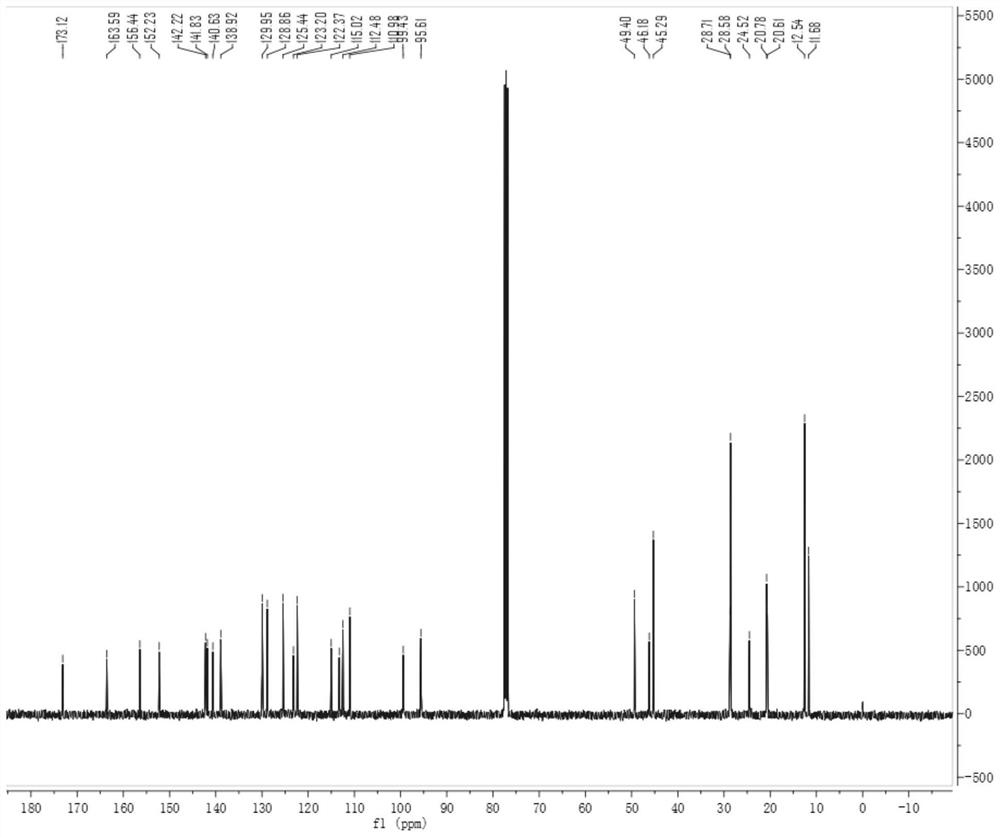
[0035] like figure 2 Probes of this invention 1 H NMR spectrum, 1 H NMR (400MHz, CDCL 3 )δ 1 HNMR (400MHz, CDCL 3 Δ8.49 (D, J = 14.5 Hz, 1H), 7.55 (S, 1H), 7.49-7.38 (m, 3H), 7.29 (S, 1H), 7.20 (D, J = 7.6 Hz, 1H), 6.80 (D, J = 8.8 Hz, 1H), 6.50 (S, 1H), 6.15 ...
Embodiment 2
[0038] Fluorescence for different concentrations of HSO 3 - And onoo - Identification
[0039] The fluorescent probe prepared in Example 1 was formulated into a DMSO mother liquor, which added different equivalents of HSO, respectively. 3 - And onoo - The solution was diluted with a phosphate buffer solution (pH = 7.4) to the concentration of 20 μm to be tested, and the fluorescence spectrum measurement was performed. like Figure 5 6. As shown, as the ion concentration to be tested, the probe exhibits a variation of fluorescence.
Embodiment 3
[0041] Fluorescence selection of different ions or molecules
[0042] The fluorescent probes prepared in Example 1 were formulated into a DMSO mother liquor, and 100 equivalents of ACO were added separately. - , F - , CL - BR - I - , CLO - , CN - , CO 3 2- , HCO 3 - HS - NO 3 - , PO 4 3- S 2 O 3 2- SO 4 2- SO 3 2- And 20 equivalents of HSO 3 - After diluting with a phosphate buffer solution (pH = 7.4) to the concentration of 20 μm to be tested, after 30 min at 37 ° C, the fluorescence spectrum was determined (λ) ex = 450 nm); According to its fluorescence intensity, the effect of different ions on fluorescence intensity of fluorescence probes is evaluated. Figure 7 a shown. Where 1-17 is 1.BLANK, 2.aco, respectively. - , 3.F - , 4.cl - , 5.br - , 6.i - , 7.Clo - , 8.cn - , 9.co 3 2- , 10.hco 3 - 11.HS - 12.no 3 - , 13.po 4 3- , 14.s 2 O 3 2- , 15.so 4 2- 16.so 3 2- 17.hso 3 - . This means that the fluorescent probe of the present invention is only HSO at 588 nm. 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com