Method for efficiently synthesizing N-methyltaurine and sodium N-methyltaurate
A technology of sodium methyl taurine and methyl taurine, which is applied in the direction of sulfonic acid preparation, sulfonate preparation, chemical instruments and methods, etc., can solve the problems of complex operation, excessive raw material surplus, harsh reaction conditions, etc. Achieve the effects of high reaction conversion rate, lower production cost, and less difficult operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
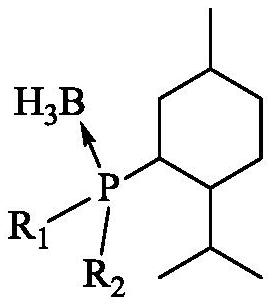
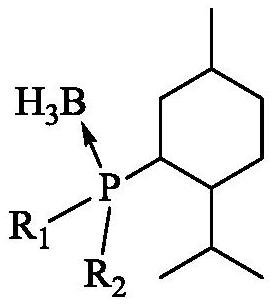
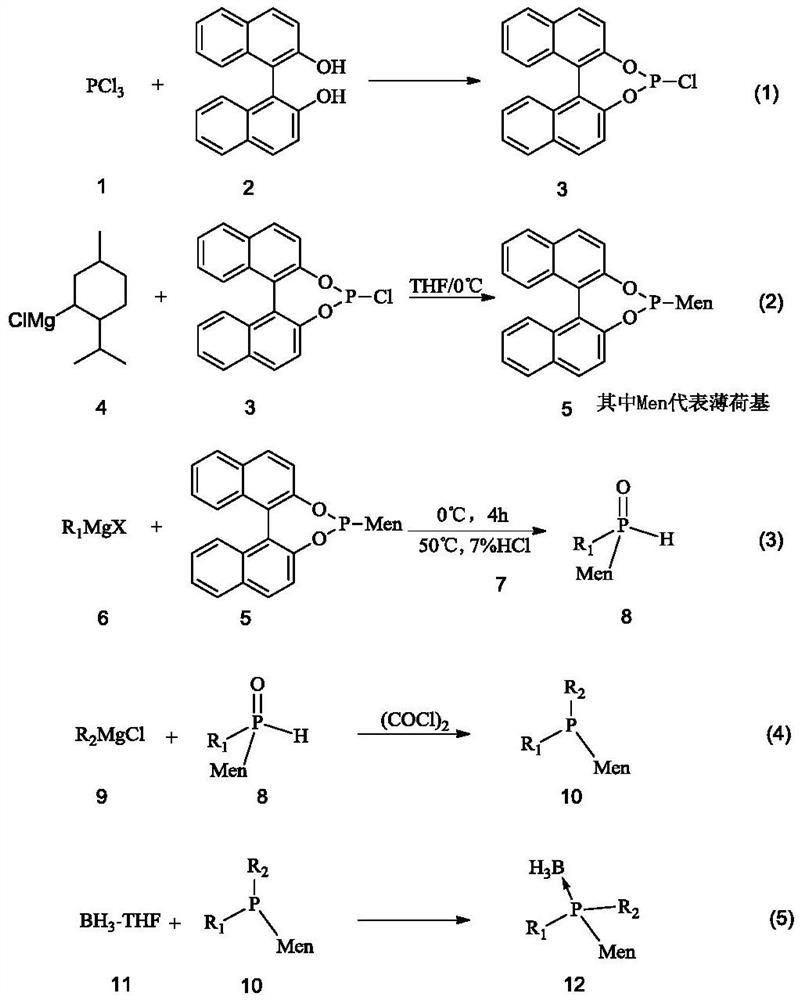
[0077] 1) Preparation of catalyst a
[0078] S1: Dissolve 229.1g of binaphthyldiol in 1L of toluene and lower the temperature to 0°C. At this temperature, add N,N-dimethylformamide (DMF) containing 329.6g of phosphorus trichloride dropwise into the solution. ) solution, the temperature was raised to 55° C. and stirred for 2 h after dripping. The toluene and DMF in the system were removed by rotary evaporation under reduced pressure, and the remaining materials were placed in a vacuum drying oven at -0.1MpaG and dried at 50°C for 12 hours to obtain a yellow fluffy compound binaphthyldioxyphosphorus chloride.
[0079] S2: at N 2 Under protection, the obtained compound was dissolved in 1.5L of tetrahydrofuran, cooled to 0°C, and 1.2L of newly prepared menthyl magnesium chloride with a concentration of 1.0mol / L was added dropwise to the system. 4h, the catalyst precursor binaphthyldioxymenthylphosphine was obtained.
[0080] S3: Under a nitrogen atmosphere, add 1.2 L of 0.8 mol...
Embodiment 2
[0114] (1) Synthesis of sodium isethionate
[0115] Add 300g of water to the reactor, add 0.68g of catalyst a, and feed SO 2 Raise the temperature until the pH=5.9, wait until the temperature of the system rises to 75°C, and add SO to the system at the same time 2 and a NaOH solution with a concentration of 15.8wt% to adjust the SO 2 and NaOH feed rate are 15.8g / min and 65.5g / min respectively, open the EO feed valve, and adjust the EO feed rate to be 11.4g / min. The system pressure at this stage is 0.2MpaG. The discharge rate of the reactor was adjusted to 92.7 g / min, and a sample was taken after 5 hours of discharge. The conversion rate of the reaction was 99.87% and the selectivity was 98.27% as measured by the IC external standard method.
[0116] NMR characterization results are 1 H NMR (CDCl 3 , 400MHz) δppm 4.11 (t, J = 7.1Hz 2H), 3.67 (s, 1H), 2.65 (t, J = 7.1Hz, 2H).
[0117] (2) Synthesis of sodium N-methyl taurate
[0118] Put 5562.3 g of the crude sodium iseth...
Embodiment 3
[0131] (1) Synthesis of sodium isethionate
[0132] Add 300g of water to the reactor, add 2.27g of catalyst b, and feed SO 2 Until PH = 6.3, raise the temperature until the temperature of the system rises to 78°C, and at the same time, feed SO into the system 2 and a NaOH solution with a concentration of 10.4wt%, adjusting the SO 2 and NaOH feed rate are 16.0g / min and 97.8g / min respectively, open the EO feed valve, and adjust the EO feed rate to be 11.3g / min. The system pressure at this stage is 0.3MpaG. The discharge rate of the reactor was adjusted to 125.1 g / min, and a sample was taken after 6 hours of discharge. The conversion rate of the reaction was 98.56% and the selectivity was 93.70% as measured by the IC external standard method.
[0133] (2) Synthesis of sodium N-methyl taurate
[0134] Put 5007.5 g of the crude sodium isethionate solution prepared in the previous step into the reactor, continue to add 372.9 g of methylamine, heat up to 150 ° C, and use N 2 Pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com