Arotinolol hydrochloride preparation method
A technology of alololol hydrochloride and molar ratio, which is applied in the field of pharmaceutical chemical industry and pharmaceutical chemistry, can solve the problems of difficult industrialization, cumbersome operation, and low yield, and achieve the effects of low cost, low reaction temperature, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
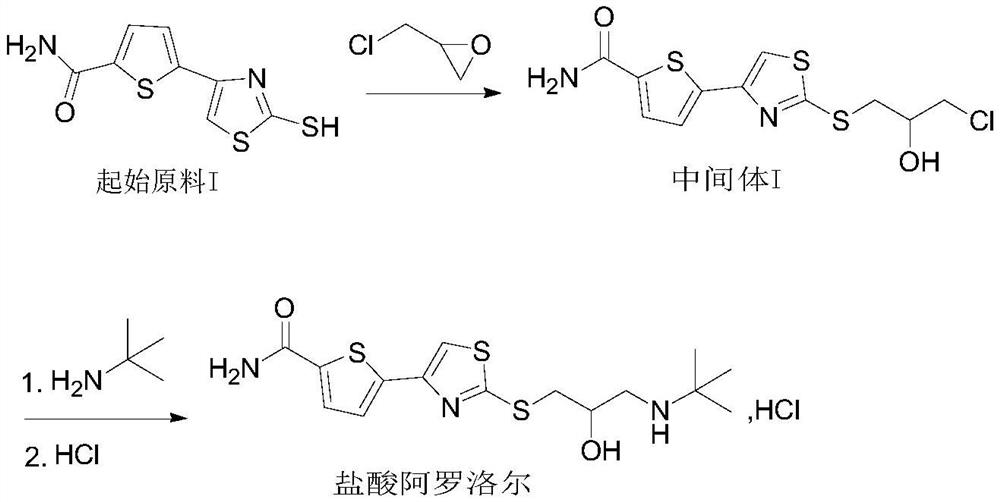
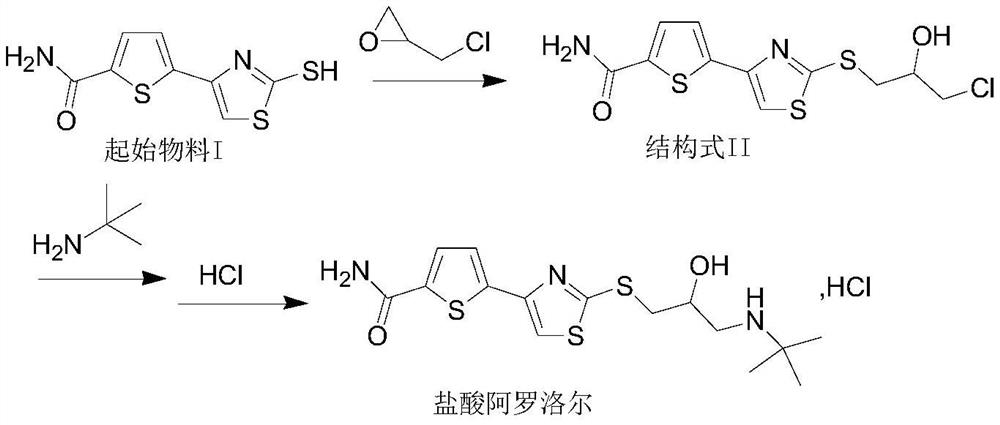
[0035] Example 1: Preparation of Alololol Hydrochloride
[0036] (a) Preparation of Intermediate I:
[0037] At room temperature, 10.0 g (41.27 mmol) of starting material I was suspended in 200 ml of water, 5.2 g (61.90 mmol) of sodium bicarbonate was added, and 3.82 g (41.29 mmol) of epichlorohydrin was added dropwise with stirring. After dropping, the temperature was raised to 45-50°C and stirred for 2h. After filtering, the filter cake was rinsed with 10 ml of water, and dried under reduced pressure at 50° C. to obtain 13.6 g (40.62 mmol) of a light yellow solid, yield 98.43%, HPLC purity: 99.57%.
[0038] ESI: [M+H] + = 335.0.
[0039] 1 H-NMR (DMSO-d6, δTMS0): 3.31~3.36ppm (1H, m), 3.51~3.55ppm (1H, m), 3.66~3.76ppm (2H, m), 4.04~4.07ppm (1H, m) , 5.80~5.81ppm (1H, d, J=4.0), 7.44 ppm (1H, br), 7.55~7.56ppm (1H, d, J=4.0), 7.72~7.73ppm (1H, d, J=4.0) , 7.99ppm (2H, br).
[0040] (b) Preparation of Alololol Hydrochloride:
[0041] Add 13.0g (38.82mmol) of intermedi...
Embodiment 2
[0044] Embodiment 2: Preparation of Alololol Hydrochloride
[0045] (a) Preparation of Intermediate I:
[0046]At room temperature, 10.0 g (41.27 mmol) of starting material I was suspended in 50 ml of water, 4.96 g (49.52 mmol) of potassium bicarbonate was added, and 11.46 g (123.87 mmol) of epichlorohydrin was added dropwise with stirring. After dropping, the temperature was raised to 25-30°C and stirred for 5h. After filtering, the filter cake was rinsed with 10 ml of purified water, and dried under reduced pressure at 50° C. to obtain 13.5 g (40.32 mmol) of a light yellow solid with a yield of 97.70% and an HPLC purity of 99.36%.
[0047] (b) Preparation of Alololol Hydrochloride:
[0048] Add 13.0g (38.82mmol) of intermediate I to 42.6g (582.44mmol) of tert-butylamine, cool down to 5°C, and add 65.0ml of ethanol dropwise. After dropping, replace with nitrogen, heat up to 55-60°C and keep stirring for 5 hours. TLC monitors, after the reaction is complete, cool down to 0...
Embodiment 3
[0049] Example 3: Preparation of Alololol Hydrochloride
[0050] (a) Preparation of Intermediate I:
[0051] At room temperature, 10.0 g (41.27 mmol) of starting material I was suspended in 400 ml of water, 8.74 g (82.50 mmol) of sodium carbonate was added, and 5.73 g (61.93 mmol) of epichlorohydrin was added dropwise with stirring. After dropping, the temperature was raised to 25-35°C and stirred for 3h. After filtering, the filter cake was rinsed with 10 ml of water, and dried under reduced pressure at 50° C. to obtain 13.3 g (39.72 mmol) of a light yellow solid, yield 96.24%, HPLC purity: 99.15%.
[0052] (b) Preparation of Alololol Hydrochloride:
[0053] Add 13.0g (38.82mmol) of intermediate I to 14.2g (194.10mmol) of tert-butylamine, cool down to 5°C, and drop in 39.0ml of isopropanol. After dropping, replace with nitrogen, heat up to 60-65°C and keep stirring for 4 hours. TLC monitors, after the reaction is complete, cool down to 0°C, slowly add 1N hydrochloric acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com