Porous nitrogen-doped carbon coaxial coated manganese dioxide nanotube and preparation method and application thereof
A technology of nitrogen-doped carbon and manganese dioxide, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve problems such as poor cycle performance, capacity loss, and low Coulombic efficiency, and achieve improved Stability, improvement of rate performance, effect of alleviating volume change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of porous nitrogen doped carbon coaxial coated manganese dioxide nanotube (MNO 2 @PNC) Negative electrode material, including the steps of: Step S1, 4.0 mmol of MNSO 4 · H 2 O, 7.0mmol KCLO 3 And 7.0mmol of CH 3 Cook dissolved in 70 ml of deionized water and continuously stirred and dispersed evenly;
[0033] Step S2, add CH to the solution obtained from S1 3 COOH adjusts pH to 3;
[0034] Step S3, the transfer to the transition from S2 in the autoclave is maintained at 200 ° C for approximately 24 h, and after filtration in a vacuum environment, the precipitate is collected, and the product is centrifuged with deionized water. Finally, at 70 ° C for 12 h;
[0035] Step 4, adding the product obtained by adding S3 to 70 ml 0.1 mol / l HCl to stir for 1 h and then dropped with 150 μl of pyrrole monomer. After stirring for 6 h, the color of the suspension was completely changed from brown to black, and after filtered in a vacuum environment The ion water washing bl...
Embodiment 2
[0044] Example 2 and Example 1 The difference is only that the sintering temperature in step S6 is 500 ° C, and the holding time is 5 h.
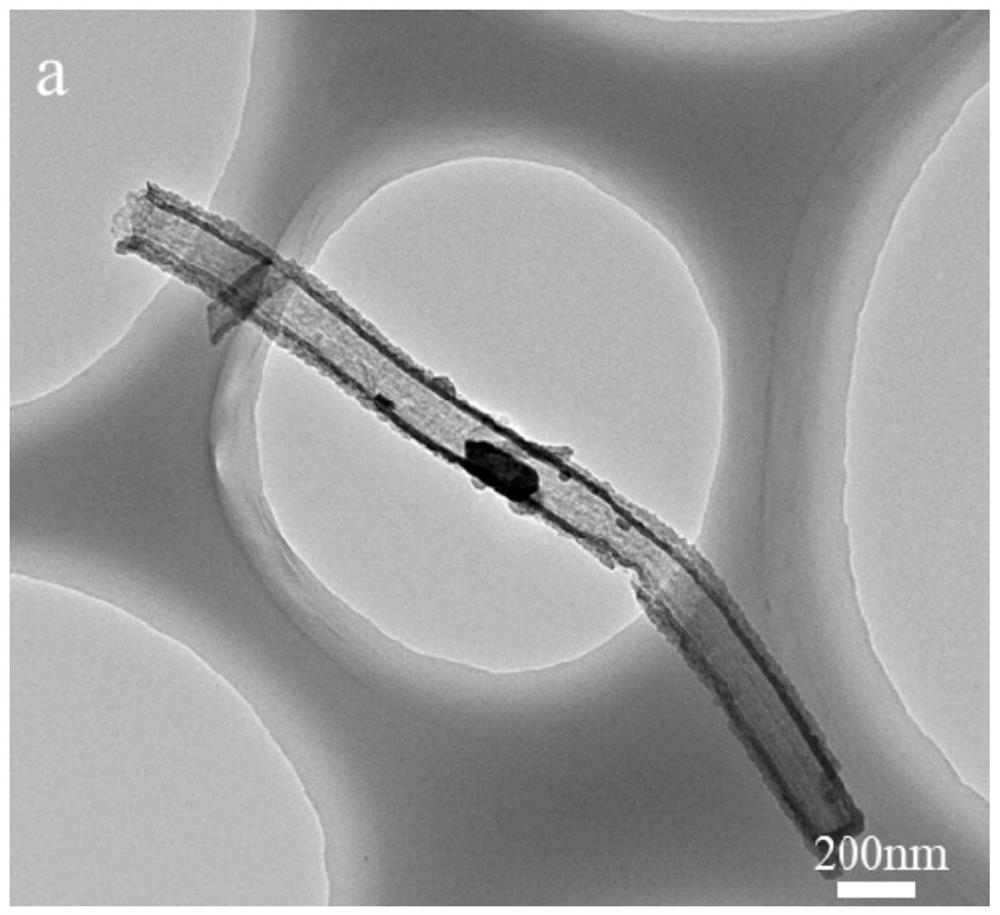
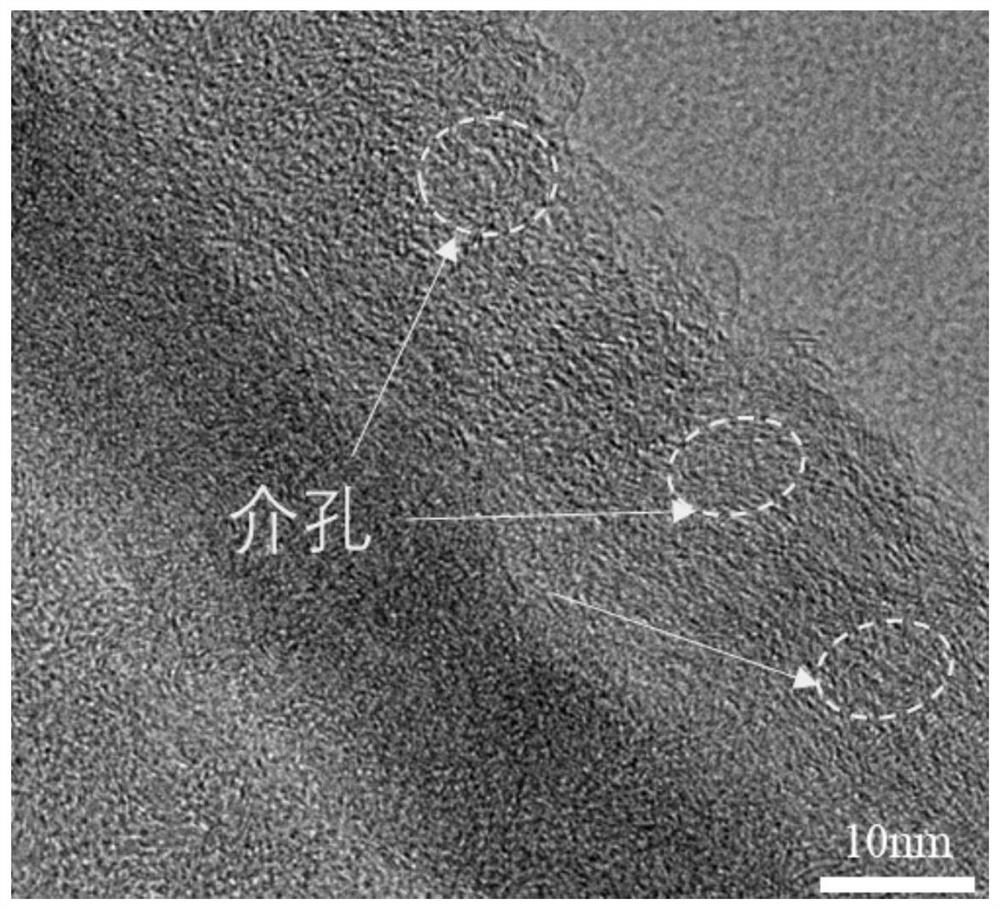
[0045] SEM, TEM, and HRTEM test and assembled battery made of Example 2 were electrochemically conductive, and the test results showed that the product MNO was prepared. 2 @PNC morphology size one; MnO obtained in this example 2 The negative electrode material of @PNC is assembled, and the lithium sheet is a positive electrode, and the buckle battery is tested. Constant current charge and discharge test shows that MNO 2 @PNC negative material assembled electrodes at 5A g -1 At current densities, the first discharge ratio is 408mAh g -1 . In 5AG -1 After the current density is 100 times, the specific capacity remains stable, which is basically consistent with Example 1, indicating the MnO 2 The cycle performance of @PNC's negative electrode is excellent.
Embodiment 3
[0047] Example 3 The difference from Example 1 is only: adding CH 3 COOH adjusts pH to 2.5, add CH 3 The amount of COOK was 7.5 mmol; the rest were basically the same as in Example 1.
[0048] The electrochemical performance tests made by SEM, TEM, and HRTEM testing and assembled cells made of Example 3 were carried out. The test results showed that the product MNO was prepared. 2 @PNC morphology size one; MnO obtained in this example 2 The negative electrode material of @PNC is assembled, and the lithium sheet is a positive electrode, and the buckle battery is tested. Constant current charge and discharge test shows that MNO 2 @PNC negative material assembled electrodes at 5A g -1 At current densities, the first discharge ratio is 407mA h g -1 . At 5A G -1 After 100 in current density, the specific capacity remains stable, which is substantially consistent with Example 1, indicating porous nitrogen doped carbon coaxial coated manganese dioxide nanotube (MNO) 2 The cycle performan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com