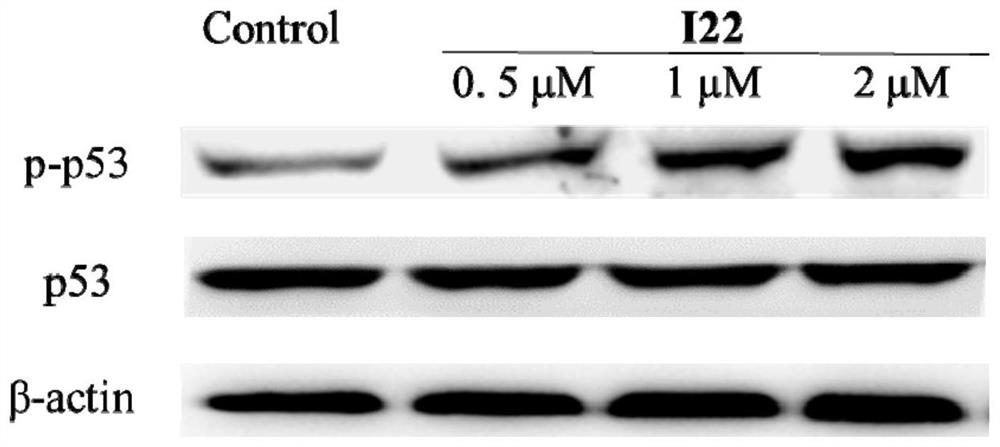
Multi-target anti-tumor small molecule and derivative, preparation method, pharmaceutical composition and application thereof
A technology of antineoplastic drugs and small molecules, applied in antineoplastic drugs, drug combinations, medical preparations containing active ingredients, etc., can solve problems such as enhanced side effects, unpredictable pharmacokinetic properties, etc., and achieve inhibition of topoisomerase activity , Low inhibitory effect on normal cells, high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of intermediate
[0040]
[0041] (1) Preparation of intermediate 2 (taking intermediate 2e as an example: R=H)
[0042] Dissolve 5.1g (37.2mmol) of reactant 1e (anthranilic acid) in 35mL of THF, add 6.6g (22.3mmol) of triphosgene (BTC) while stirring at 0°C, and react the mixture at 35°C for 12h, and the reaction ends After filtration, filter cake with Et 2 O wash (20 mL x 3) afforded a brown solid (Intermediate 2e).
[0043] 1 H NMR (600MHz, DMSO-d 6 )δ11.73(s,1H),7.91–7.89(m,1H),7.74–7.71(m,1H),7.25–7.22(m,1H),7.15(d,J=8.0Hz,1H)ppm.
[0044] (2) Preparation of intermediate 3 (taking intermediate 3e as an example: R=H)
[0045] 3.8g (23.3mmol) of intermediate 2e and hydrazine hydrate (85%) were successively added into a 50mL round-bottomed flask, refluxed for 3h, and the reaction process was detected by thin-layer chromatography. After the reaction was completed, it was cooled to 0° C. and filtered, and the filter cake was washed...
Embodiment 2
[0053] Embodiment 2: the preparation of compound I7
[0054] Intermediate 6a 1 (400mg, 0.88mmol) was dissolved in 15mL of THF, and lithium hydroxide (92.4mg, 2.2mmol) aqueous solution (5mL) was added dropwise, stirred at room temperature overnight, and the solvent was removed under reduced pressure to obtain a milky white solid, which was dissolved in 30mL of water and washed with 1M Adjust the pH to 5 with hydrochloric acid solution, filter to obtain a light yellow solid, dry it and stir it with TBTU (340.4mg, 1.06mmol) in DMF (10mL) solution at room temperature for 5min, add 115.4mg (1.14mmol) triethylamine and continue stirring for 2min, then Add 285.4mg (2.64mmol) of o-phenylenediamine and react at 35°C for 2h. The reaction solution was concentrated, and the concentrated solution was separated by silica gel column chromatography, and the eluent was CH 2 Cl 2 Mix solvent with MeOH (30:1) to obtain dark red solid 135.3 mg, yield: 30%.
[0055] 1 H NMR (600MHz, DMSO-d 6...
Embodiment 3
[0056] Embodiment 3: the preparation of compound I8
[0057] with intermediate 6a 2 instead of intermediate 6a 1 Prepared with reference to the method described in Example 1, a dark red solid was obtained with a yield of 35%.
[0058] 1 H NMR (600MHz, DMSO-d 6 )δ9.12(s,1H),8.80–8.77(m,1H),8.27(d,J=7.8Hz,1H),8.24(d,J=2.3Hz,1H),7.94–7.87(m,3H ),7.65(d,J=8.7Hz,1H),7.56(t,J=5.1Hz,1H),7.15(d,J=7.8Hz,1H),6.89–6.87(m,1H),6.72–6.70 (m,1H),6.53–6.50(m,1H),4.82(s,2H),3.51–3.48(m,2H),2.39(t,J=7.4Hz,2H),1.82–1.77(m,2H ),1.73–1.68(m,2H),1.51–1.46(m,2H)ppm; 13 C NMR (150MHz, DMSO–d 6 ): δ171.7, 156.7, 149.2, 145.1, 144.1, 142.4, 136.9, 133.5, 132.7, 129.8, 128.9, 128.7, 126.7, 126.2, 125.8, 124.1, 123.4, 122.0, 121.6, 1174.1, 113 28.2,26.9,25.7ppm.HRMS(ESI)m / z calcd for C 27 h 25 BrN 6 o 2 [M+H] + :545.1295,found:545.1309.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com