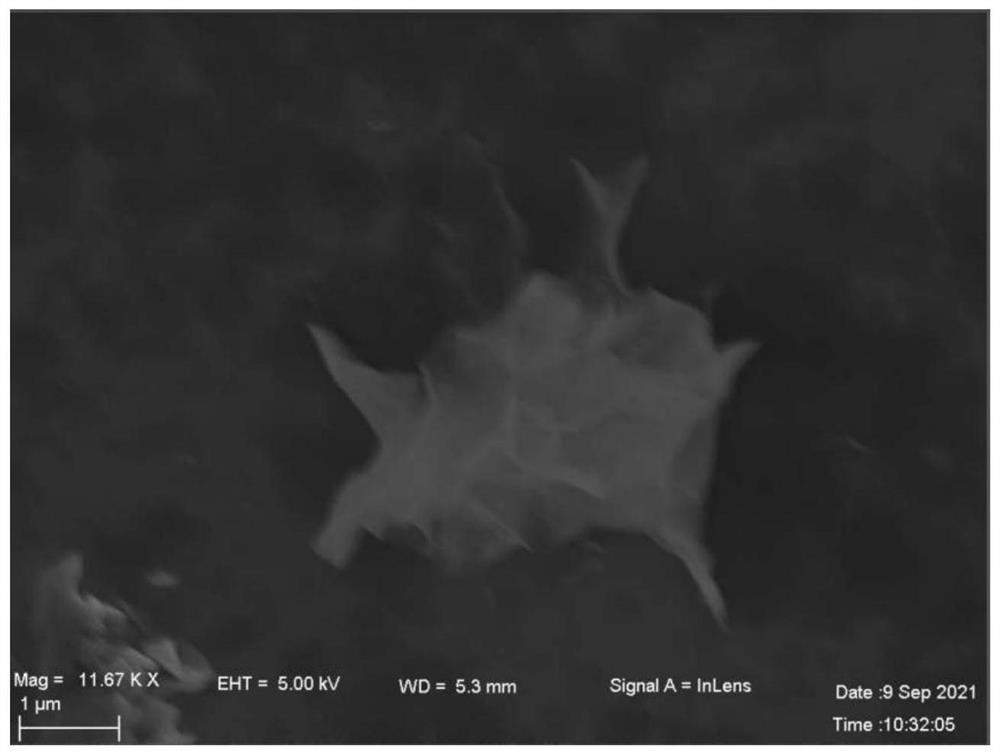
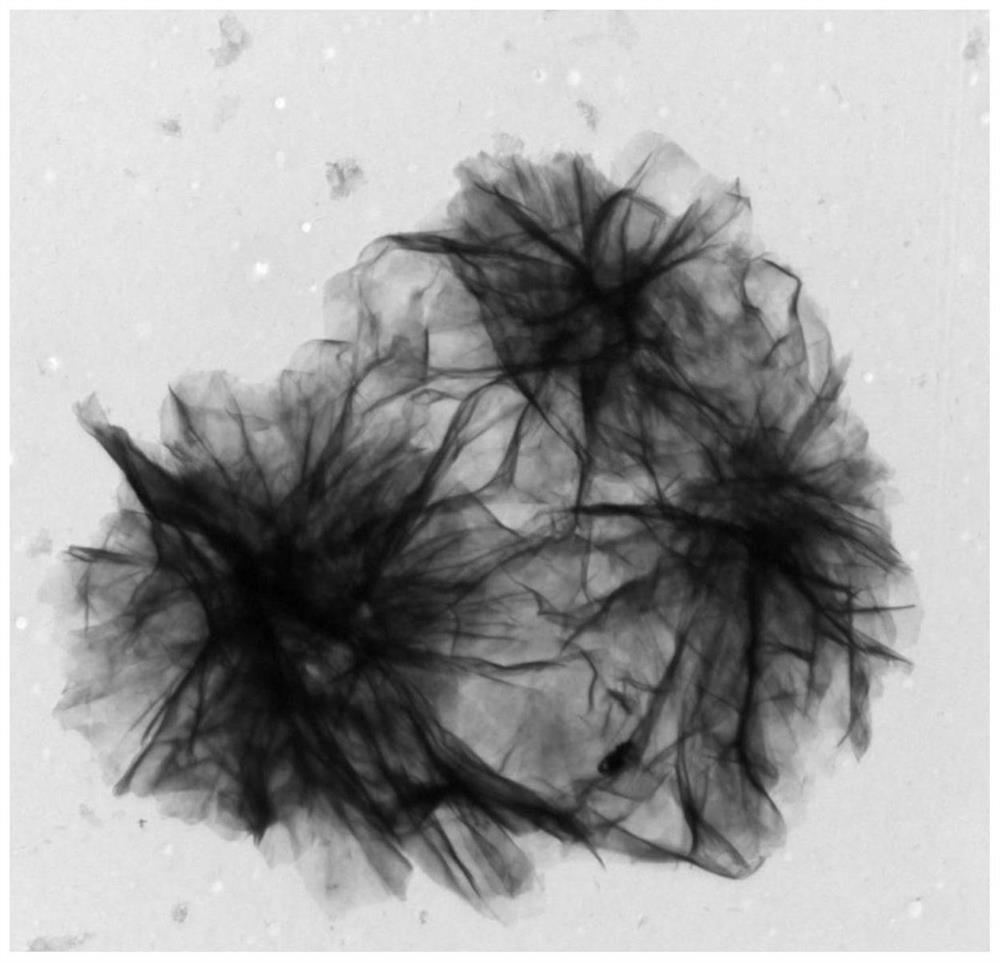
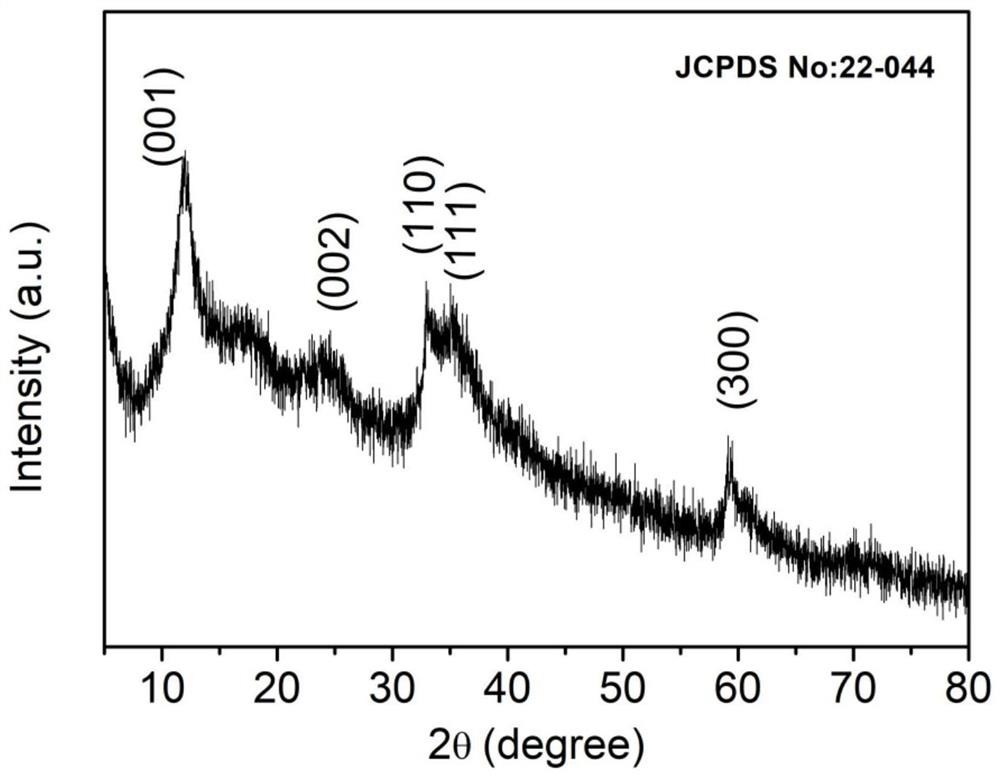
Ni-Cu LDH electrocatalyst with nanoflower structure, preparation method and application thereof
An electrocatalyst and nanoflower technology, applied in electrodes, electrolysis components, electrolysis process, etc., can solve the problem of difficult application of catalysts, and achieve the effects of high BET, excellent structure, electrochemical durability, and high catalytic efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Dissolve nickel nitrate hexahydrate in a mixed solution of ionized water and triethylene glycol, and mechanically stir to obtain a uniform solution A; the mass ratio of ionized water to triethylene glycol is 1:4; nitric acid hexahydrate The molar ratio of nickel to deionized water is 1:5;
[0029] (2) Add urea and copper chloride dihydrate to the uniform solution A obtained in step (1) to obtain a uniform solution B; in solution B, the molar ratio of urea, copper chloride dihydrate and nickel nitrate hexahydrate is 2:5 :0.5;
[0030] (3) The homogeneous solution B was hydrothermally synthesized at 120°C for 18-30h, after the reaction, centrifuged at 4000r / min for 100min, filtered and washed with deionized water and absolute ethanol several times, and dried in vacuum at 60°C 24h to obtain Ni-CuLDH electrocatalyst.
Embodiment 2
[0032] (1) Dissolve nickel nitrate hexahydrate in a mixed solution of ionized water and triethylene glycol, and mechanically stir to obtain a uniform solution A; the mass ratio of ionized water to triethylene glycol is 1:5; nitric acid hexahydrate The molar ratio of nickel to deionized water is 1:5;
[0033] (2) Add urea and copper chloride dihydrate to the uniform solution A obtained in step (1) to obtain a uniform solution B; in solution B, the molar ratio of urea, copper chloride dihydrate and nickel nitrate hexahydrate is 2:5 :0.5;
[0034] (3) The homogeneous solution B was hydrothermally synthesized at 120°C for 18-30h, after the reaction, centrifuged at 4000r / min for 100min, filtered and washed with deionized water and absolute ethanol several times, and dried in vacuum at 60°C 24h to obtain Ni-CuLDH electrocatalyst.
Embodiment 3
[0036] (1) Dissolve nickel nitrate hexahydrate in a mixed solution of ionized water and triethylene glycol, and mechanically stir to obtain a uniform solution A; the mass ratio of ionized water to triethylene glycol is 1:7; nitric acid hexahydrate The molar ratio of nickel to deionized water is 1:5;
[0037] (2) Add urea and copper chloride dihydrate to the uniform solution A obtained in step (1) to obtain a uniform solution B; in solution B, the molar ratio of urea, copper chloride dihydrate and nickel nitrate hexahydrate is 2:5 :0.5;
[0038] (3) The homogeneous solution B was hydrothermally synthesized at 120°C for 18-30h, after the reaction, centrifuged at 4000r / min for 100min, filtered and washed with deionized water and absolute ethanol several times, and dried in vacuum at 60°C 24h to obtain Ni-CuLDH electrocatalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com