Application of wedelolactone in preparation of medicine and health care product for preventing and/or treating herpes virus infection
A technology of herpes virus infection and wecarolide, which is applied in the field of medicine and achieves the effects of inhibiting virus proliferation, interfering with virus adsorption, and having good market application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
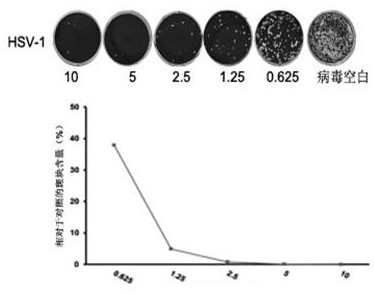
[0035] Embodiment 1: the effect of wedelolide inhibiting herpes simplex virus in vitro
[0036] 1. Experimental method
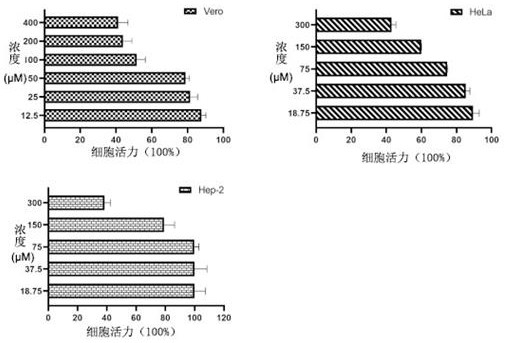
[0037] 1.1 Cytotoxicity test of wedelolide
[0038] The cytotoxicity of wedelolide was determined on three different cells. Three kinds of cells, Vero, Hela, and Hep-2, were inoculated in 96-well plates until they were covered with a single layer. 50 μM, 25 μM, 12.5 μM or 300 μM, 150 μM, 175 μM, 37.5 μM, 18.75 μM) were added to 96-well plate, and each concentration was replicated three times, and a blank control group was set at the same time. Cells were placed at 37°C in 5% CO 2 After culturing in a constant temperature cell incubator for 24 hours, discard the drug, add 4% paraformaldehyde to fix at room temperature for 15 minutes, absorb and discard, add 5% crystal violet to stain at room temperature for 15 minutes, wash and dry, and measure A with a microplate reader. 540nm value.
[0039] Cell survival rate = test group A 540nm / Negative control g...
Embodiment 2
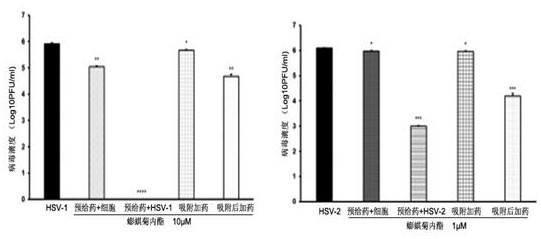
[0058] Embodiment 2: the mechanism of action of wedelolide against HSV
[0059] 1. Experimental method
[0060] 1.1 Indirect immunofluorescence test
[0061] Inoculate Vero cells on a 3.5 cm glass-bottom dish, and when the cells grow to a density of 30% to 40%, treat HSV-1 with wedelolide (final concentration 10 μM) according to the four different modes of action in 1.2 of Example 1 (MOI= 1), cultured in a 37°C incubator for 8 hours; then washed the cells with PBS, fixed with 4% paraformaldehyde at room temperature for 15 minutes, washed with PBS for 2 minutes, permeabilized with 0.25% Triton X-100 for 10 minutes, washed with PBS for 2 minutes, and washed with 2% BSA in Block at 37°C for 1 hour, wash with PBS three times, add ICP5 antibody (1:100 dilution) and incubate at 4°C for 16 hours, add FITC-coupled secondary antibody and incubate at 4°C for 4 hours after washing, wash three times, treat with DAPI for 10 minutes, and perform co-treatment after washing. Images were foc...
Embodiment 3
[0083] Example 3: The effect of Werethrin on HSV-infected mice
[0084] 1. Experimental method
[0085] 1.1 Experimental modeling
[0086] All the mice used in the present invention conform to the guidelines for humane treatment of experimental animals of the Ministry of Science and Technology of China (vgkfcz-2006-398). BALB / c female mice aged 3-4 weeks, weighing 14-15g, were randomly divided into 5 groups: blank control group, virus control group, positive drug (aciclovir 10 mg / kg / day) group, and the The high-dose (5 mg / kg / day) group of esters and the low-dose (2.5 mg / kg / day) group of isthrin. The drug was inoculated by nasal drip, and the drug was administered 4 hours after the drug was administered, and the drug was administered continuously for 5 days.
[0087] 1.2 Mouse detection
[0088] The mice were dissected 72 hours after exposure, and the lungs and spinal cords of the mice were taken to verify the anti-HSV-1 effect of the drug in vivo by plaque test and real-ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com