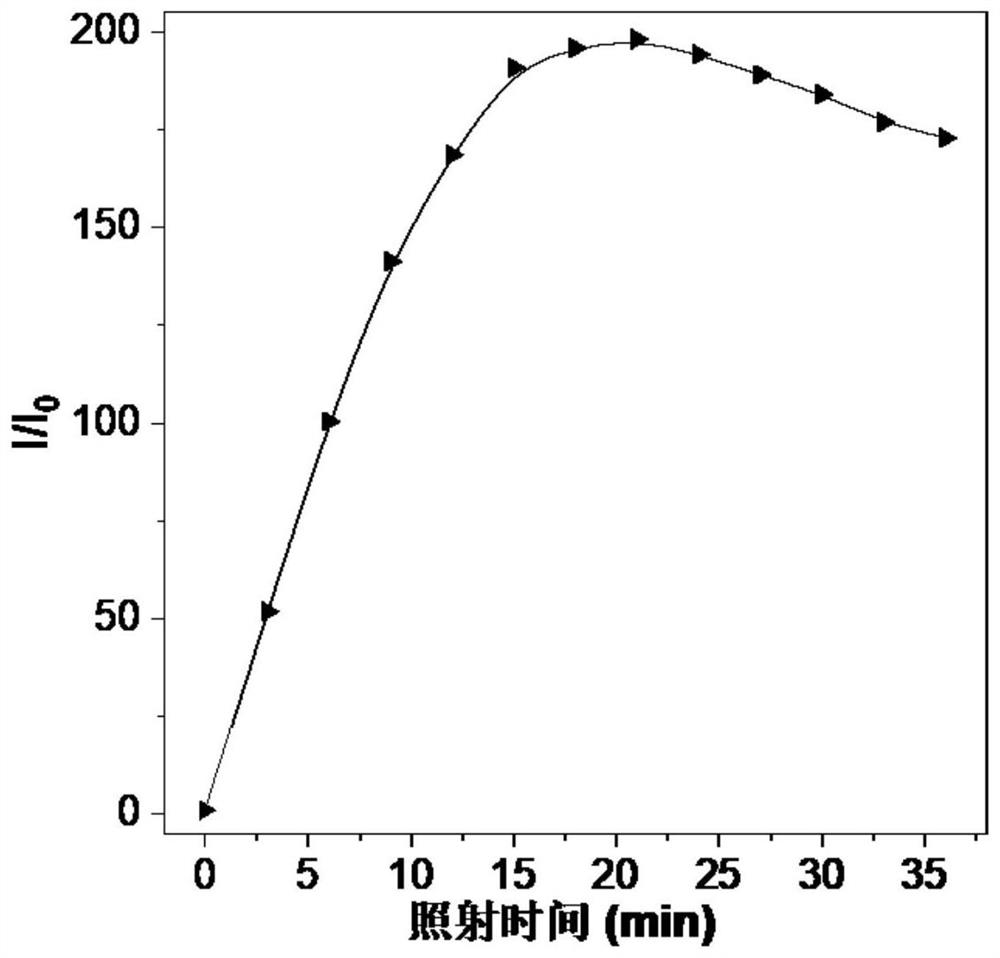
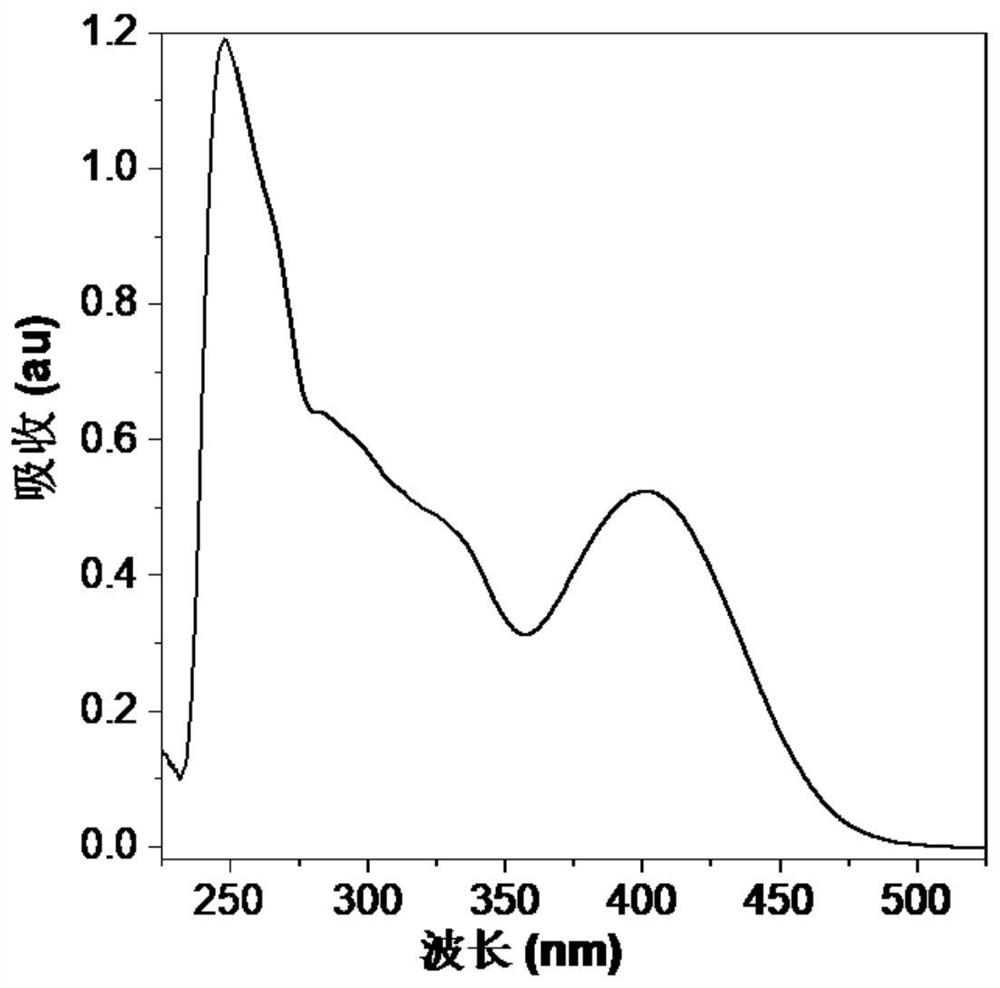
Squaric acid derivative, maleic anhydride derivative and maleimide biological coupling material as well as preparation methods and applications thereof
A technology of squaraine derivatives and maleimide, applied in the field of biomaterial synthesis, can solve the problems of low reactivity, complex pre-modification, difficult synthesis of raw materials, etc., and achieve the effect of overcoming no fluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] A squaraine derivative (SQ-PhOCH 3 ) Preparation of materials
[0062]
[0063] The synthetic route is as follows:
[0064]
[0065] a) 3,4-dichlorocyclo-3-ene-1,2-dione (2): Add squaraine (1g, 8.78mmol) in a 100ml two-necked flask, then add thionyl chloride (10mL, 137mmol ), heated to 60°C, then added 1mL of N,N-dimethylformamide, and reacted for 6 hours. After the reaction was completed, the excess thionyl chloride was removed by a rotary evaporator, the product was collected, and the next reaction was carried out directly.
[0066] b) Synthesis of 3,4-bis(4-methoxyphenyl)cyclo-3-ene-1,2-dione (3) (SQ-PhOCH 3 ): Add aluminum trichloride (2.58g, 19.32mmol) in a 100ml two-necked bottle, nitrogen-pump-nitrogen cycle 3 times (10 minutes each time), and then dissolve the compound (2) obtained in step (a) In 20ml of dichloromethane, inject into the reaction vial. Then anisole (2.09 g, 13.93 mmol) was added, heated to 55°C, and reacted for 5 hours. After the react...
Embodiment 2
[0068] Preparation of a squaraine derivative (SQ-TPA) material
[0069]
[0070] The synthetic route is as follows:
[0071]
[0072] Synthesis of 3,4-bis(4-(diphenylamino)phenyl)cyclo-3-ene-1,2-dione (SQ-TPA): Add aluminum trichloride (2.58g, 19.32 mmol) and triphenylamine (4.73g, 19.32mmol), nitrogen-pumping-nitrogen circulation 3 times (10 minutes each time), then the compound (2) obtained in step (a) in Example 1 was dissolved in 20mL Chloromethane, injected into the reaction flask, then added 40mL, heated to 55°C, and reacted for 5 hours. After the reaction was completed, it was extracted with dichloromethane / water (the volume ratio of the dichloromethane to water was 1 / 1), dried over anhydrous magnesium sulfate for 2 hours, separated and purified by column chromatography to obtain red pink (3,49g) , the yield was 70%. 1 H NMR (500MHz, CDCl 3 )δ7.98(d, J=8.9Hz, 2H), 7.34(t, J=7.9Hz, 4H), 7.23–7.08(m, 6H), 7.03(d, J=8.9Hz, 2H).
Embodiment 3
[0074] Preparation of a squaraine derivative (SQ-CZ) material
[0075]
[0076] The synthetic route is as follows:
[0077]
[0078] Synthesis of 3,4-bis(9-phenyl-9H-carbazol-3-yl)cyclobutene-1,2-dione (SQ-CZ): Add aluminum trichloride (2.58g , 19.32mmol) and triphenylamine (4.69g, 19.32mmol), nitrogen-pumping-nitrogen circulation 3 times (each 10 minutes), then the compound (2) obtained in step (a) in embodiment 1 was dissolved in Inject 20mL of dichloromethane into the reaction flask, then add 40mL, heat to 55°C, and react for 5 hours. After the reaction was completed, it was extracted with dichloromethane / water (the volume ratio of the dichloromethane to water was 1 / 1), dried over anhydrous magnesium sulfate for 2 hours, separated and purified by column chromatography to obtain yellow pink (3,52g) , the yield was 71%. 1 H NMR (400MHz, CDCl 3 )δ9.10,9.10,8.25,8.25,8.23,8.23,8.20, 8.18,7.68,7.66,7.64,7.60,7.60,7.58,7.56,7.54,7.52,7.50,7.48,7.46,7.44,7.42,7.37,7.37 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com