Methyl 4-fluoro-5-hydroxy-2-nitrobenzoate
A technology of methyl nitrobenzoate and nitrobenzoic acid, which is applied in the field of preparation of methyl 4-fluoro-5-hydroxy-2-nitrobenzoate, can solve the problem of many side reactions and is not suitable for large-scale production , high energy consumption and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
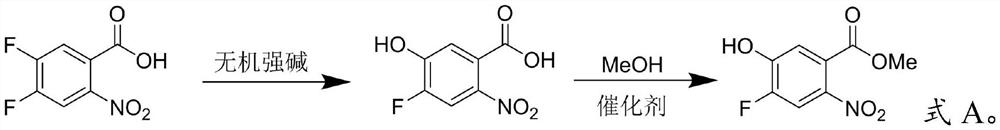
[0022] The invention provides a kind of preparation method of 4-fluoro-5-hydroxyl-2-nitrobenzoic acid methyl ester, comprising the following steps:
[0023] (1) Mix 4,5-difluoro-2-nitrobenzoic acid, an inorganic strong base and a polar solvent, and perform a substitution reaction to obtain 4-fluoro-5-hydroxyl-2-nitrobenzoic acid;
[0024] (2) Mix the 4-fluoro-5-hydroxyl-2-nitrobenzoic acid, methanol and a molecular sieve catalyst for esterification to obtain methyl 4-fluoro-5-hydroxyl-2-nitrobenzoate.
[0025] The invention mixes 4,5-difluoro-2-nitrobenzoic acid, inorganic strong base and polar solvent to carry out substitution reaction to obtain 4-fluoro-5-hydroxyl-2-nitrobenzoic acid. In the present invention, the strong inorganic base is preferably one or more of sodium hydroxide, potassium hydroxide, cesium hydroxide, lithium hydroxide and rubidium hydroxide. In the present invention, the molar ratio of the 4,5-difluoro-2-nitrobenzoic acid to the strong inorganic base is ...
Embodiment 1
[0053] (1) Synthesis of 4-fluoro-5-hydroxyl-2-nitrobenzoic acid
[0054]
[0055] 10 g of 4,5-difluoro-2-nitrobenzoic acid and 13 mL of water were added to the reaction kettle to form a suspension, and 20 mL of 30% NaOH aqueous solution was added dropwise. After the dropwise addition was completed, the mixture was heated to 80° C. for 30 minutes, and the reaction progress was monitored by TCL. Cool to room temperature after the reaction, adjust the pH to 1 with hydrochloric acid, extract with 25 mL of ethyl acetate, add 20 g of anhydrous sodium sulfate to dry the organic phase at the end of the extraction; perform rotary evaporation at 35 °C to remove the extractant to obtain a crude product. Recrystallize with 25 mL of toluene:cyclohexane (1:1) mixed solution to obtain 4-fluoro-5-hydroxy-2-nitrobenzoic acid. The yield was 95%, and the purity by HPLC was 99%.
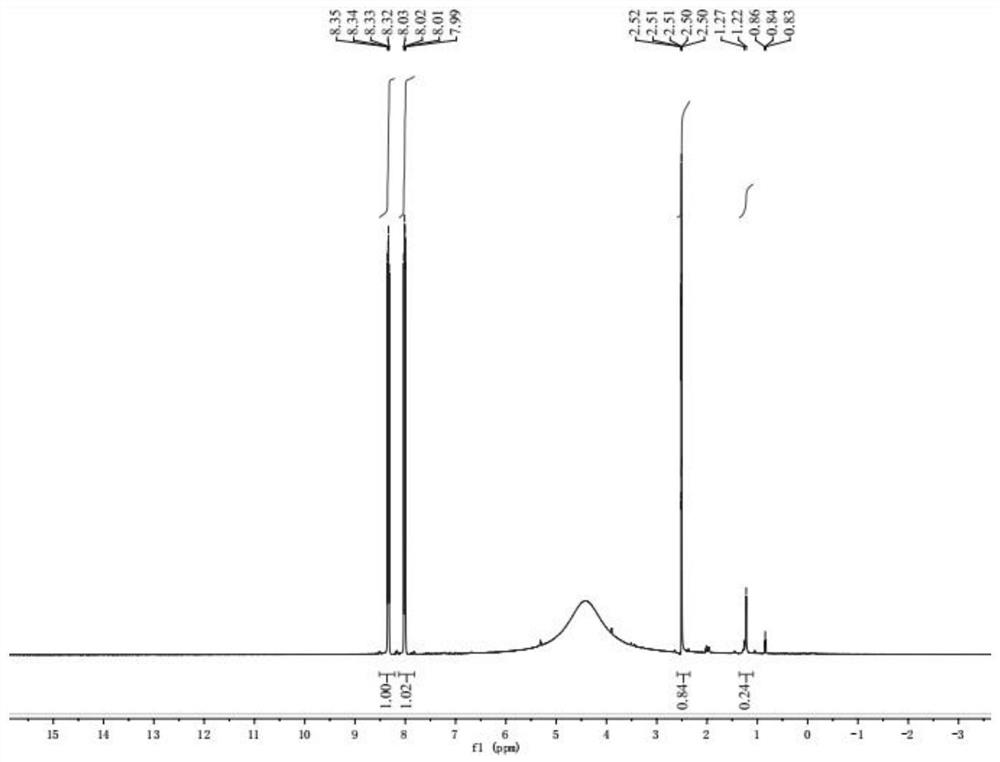
[0056] The obtained 4-fluoro-5 hydroxy-2-nitrobenzoic acid 1 H-NMR picture as figure 1 shown.
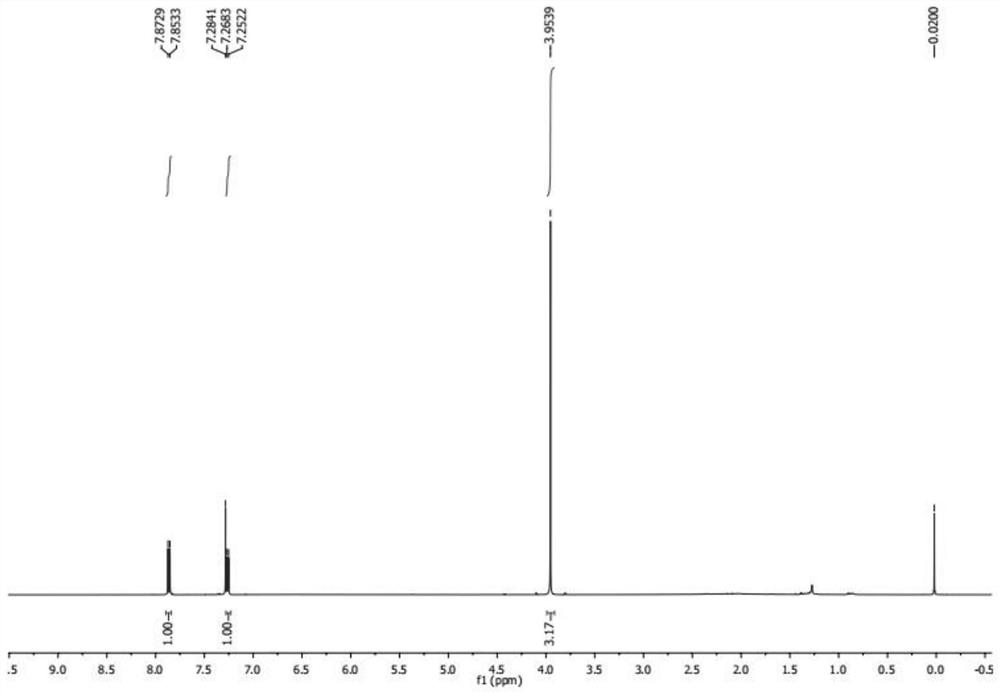
[0057] (2) Sy...
Embodiment 2
[0062] (1) Synthesis of 4-fluoro-5-hydroxyl-2-nitrobenzoic acid
[0063]
[0064] Add 20g of 4,5-difluoro-2-nitrobenzoic acid and 30mL of methanol into the reaction kettle to mix, and add 40mL of 20% KOH aqueous solution dropwise. After the dropwise addition was completed, the mixture was heated to 85° C. for 20 minutes, and the reaction progress was monitored by TCL. Cool to room temperature after the reaction, adjust the pH to 3 with hydrochloric acid, extract with 70 mL of ethyl acetate, add 40 g of anhydrous magnesium sulfate to dry the organic phase after the extraction, and perform rotary evaporation at 37 ° C to remove the extractant to obtain a crude product. Recrystallize with 30 mL of toluene:cyclohexane (1:1) mixed solution to obtain 4-fluoro-5-hydroxy-2-nitrobenzoic acid. The yield was 93%, and the purity by HPLC was 98.6%.
[0065] (2) Synthesis of methyl 4-fluoro-5-hydroxy-2-nitrobenzoate
[0066]
[0067] Dissolve 15g of 4-fluoro-5-hydroxy-2-nitrobenzoi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com