Expression vector, recombinant adeno-associated virus and application of recombinant adeno-associated virus in preparation of novel 2019 coronavirus vaccine
A coronavirus and expression vector technology, used in the preparation of 2019 new coronavirus vaccine applications, can solve the lack of long-acting research on AAV new crown vaccine and other problems, and achieve reduced adverse reactions, good stability, and antibody titers. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063]The present invention also provides a preparation method of recombinant adeno-associated virus, comprising the following steps: co-incubating the above-mentioned expression vector, helper plasmid pHelper and serotype plasmid pRepCap, and transfecting host cells in the presence of transfection reagent polyethyleneimine After the cells are cultivated, the cells are collected by centrifugation, lysed and purified to obtain a purified solution containing recombinant adeno-associated virus.
[0064] In some embodiments, the serotype plasmid pRepCap is pRep2Cap6, wherein the nucleotide sequence of the cap gene is shown in SEQ ID No.7.
[0065] The preparation of the recombinant adeno-associated virus can adopt the methods known to those skilled in the art. In the embodiment of the present invention, HEK293T-based AAV helper-free system (AAVHelper-FreeSystem) is used for production, that is, the three-plasmid co-transfection method. The AAV helper-free system contains expressio...
Embodiment 1
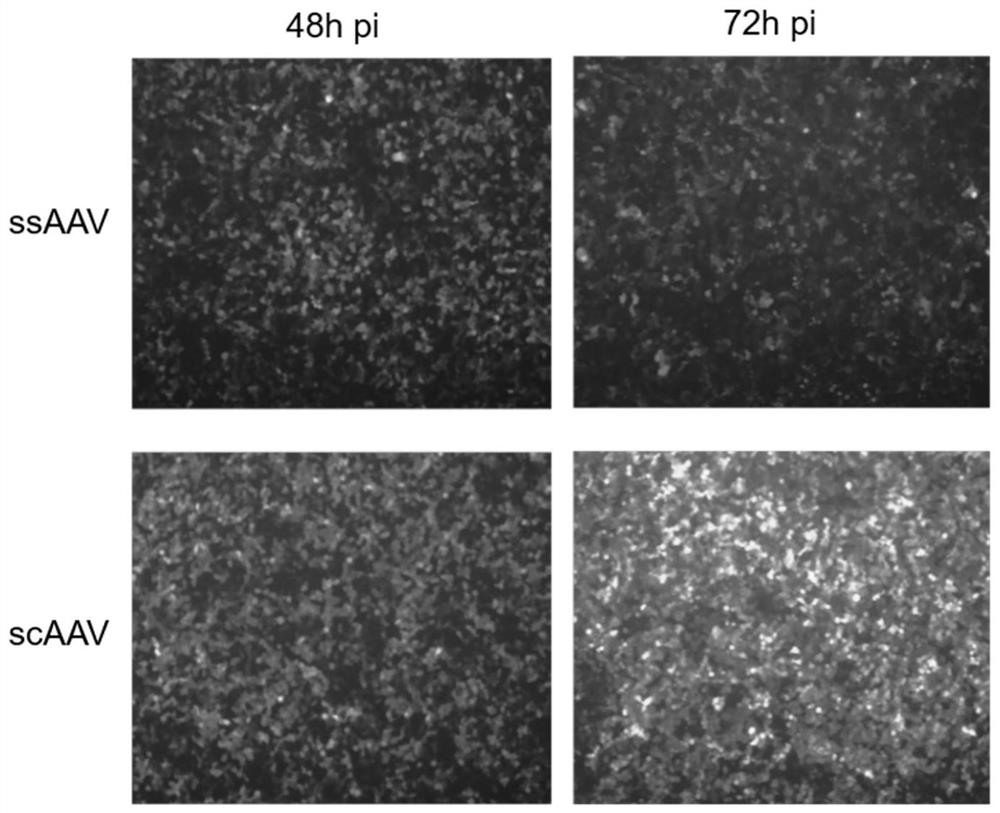
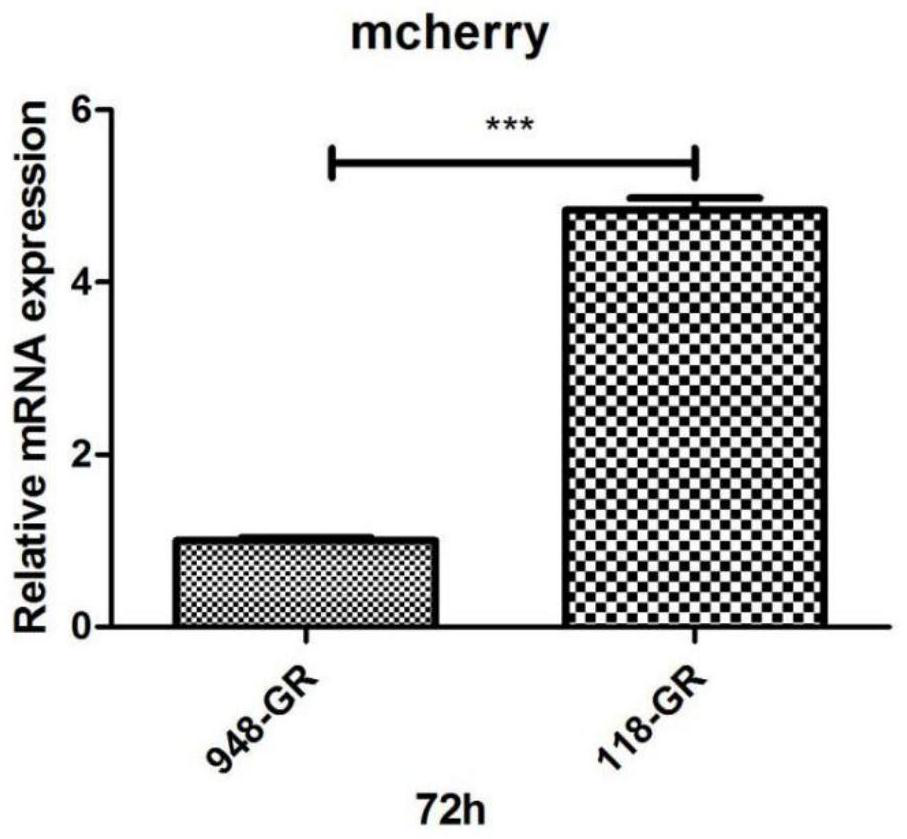
[0075] Single-stranded AAV is used in the prior art to express the new crown vaccine molecule. This example verifies the difference in the expression levels of single- and double-stranded viruses in cells.
[0076] 1. Plasmid construction
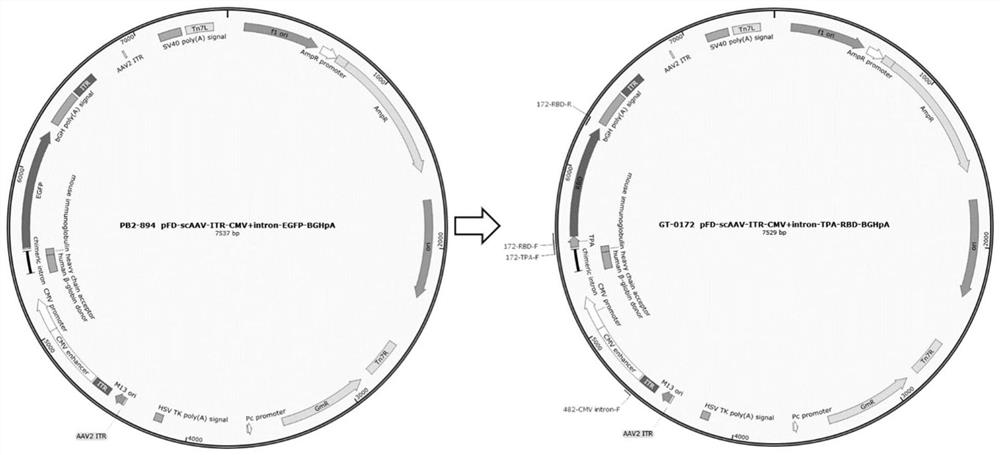
[0077] Two kinds of rAAV plasmids, rAAV-CMV-mCHERRY-BGHpA (ID: PB2-0948) and pFD-scAAV-CMV-mCHERRY-BGHpA (ID: GT-0118), were constructed for the preparation of single-chain AAV and double-chain AAV, respectively.
[0078] 2. Virus packaging and titer detection
[0079] HEK293T cells were treated with 1.5×10 per dish 24 hours before transfection 6 The density of cells was inoculated in a 100mm culture dish, and the core plasmid pAAV-GOI (PB2-0948 or GT-0118): serotype plasmid pRepCap: helper plasmid pHelper=5μg: 10μg: 7.5μg and 45μL transfection reagent polyethylene glycol were added. Amine solution (PEI, 1 mg / mL) was used to incubate the transfection. After 72 hours of transfection, the cells were collected by centrifugation, lysed and pu...
Embodiment 2
[0086] Embodiment 2 Preparation of different antigen molecule carriers
[0087] The SARS-CoV-2 (2019-nCoV-WIV04) full-length S protein coding sequence used in this example is the nucleotide sequence shown in SEQ ID No. 1 commissioned by GenScript Company. The sequence was optimized with human codons and named 2019-HnCOV-S. Select the main antigen recognition epitope RBD region of S protein, as shown in SEQ ID No.2, and name it RBD; the amino acid sequence corresponding to this sequence is shown in SEQ ID No.3. On the basis of this sequence, the following signal peptide TPA and three targeting peptide sequences were designed, as shown in Table 1.
[0088] Table 1 Signal peptide and targeting peptide sequence information
[0089]
[0090] Wherein TPA is a secretion signal peptide, added to the N-terminal of the RBD sequence when the vector is constructed, and targeting peptides are respectively added to the C-terminal of the RBD sequence, and the targeting peptides used in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com