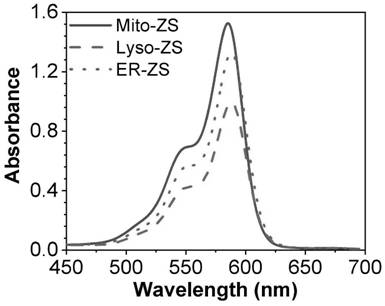
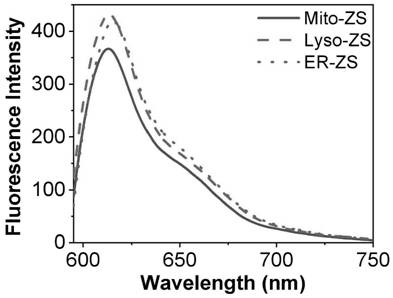
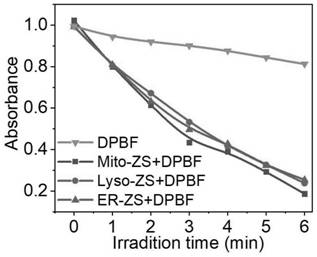
Organelle targeting photosensitizer capable of activating pyroptosis of tumor cells and preparation method and application thereof
A technology of tumor cells and photosensitizers, applied in the application of photodynamic therapy and enhanced tumor immunotherapy, organelle targeting photosensitizers and their preparation fields, can solve problems such as apoptosis, and achieve fewer synthesis steps and excellent targeting performance. , the effect of raw material economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of mitochondria-targeted photosensitizer Mito-ZS:
[0067] (1) Synthesis of Compound 1
[0068]
[0069] 2,3,3-Trimethyl-3H-benzo[g]indole (3.57g, 17mmol) and ethyl iodide (3.21g, 20.6mmol) were dissolved in 15mL of acetonitrile and refluxed at 90°C overnight. The reaction was detected with a TCL plate. After the reaction was complete, the precipitate was cooled to room temperature and filtered, washed with ether for 3 times, and dried to obtain compound 1 with a yield of 81.18%.
[0070] (2) Synthesis of compound Mito-ZS
[0071]
[0072] Compound 1 (300 mg, 0.82 mmol) and 3,5-diiodo-4-hydroxybenzaldehyde (368 mg, 0.98 mmol) were dissolved in 16 mL of absolute ethanol, and 3 drops of piperidine (150 μL) were added dropwise. The reaction was carried out overnight at room temperature. After the reaction, the precipitate was filtered, washed with ice-absolute ethanol for 3 times, and dried to obtain the compound Mito-ZS with a yield of 50%.
Embodiment 2
[0074] Preparation of lysosome-targeted photosensitizer Lyso-ZS:
[0075] (1) Synthesis of Compound 2
[0076]
[0077] 2,3,3-Trimethyl-3H-benzo[g]indole (1.06g, 5.08mmol) and p-bromomethylbenzoic acid (1.21g, 5.59mmol) were dissolved in 24mL of acetonitrile, at 85°C Reflux overnight. The reaction was detected with a TCL plate. After the reaction was complete, the precipitate was filtered after cooling to room temperature, washed with cold acetone for 3 times, and dried to obtain compound 2 with a yield of 40%.
[0078] (2) Synthesis of Compound S-1
[0079]
[0080] Compound 2 (200 mg, 0.47 mmol) and 3,5-diiodo-4-hydroxybenzaldehyde (211 mg, 0.56 mmol) were dissolved in 10 mL of absolute ethanol, and 3 drops of piperidine (150 μl) were added dropwise. The reaction was carried out overnight at room temperature. After the reaction, the precipitate was filtered, washed with ice-absolute ethanol three times, and dried to obtain compound S-1 with a yield of 51%.
[0081]...
Embodiment 3
[0085] Preparation of endoplasmic reticulum targeting photosensitizer ER-ZS:
[0086] (1) Synthesis of compound 3
[0087]
[0088] Dissolve ethylenediamine (1.5g, 25mmol) in 100mL of dichloromethane, then dissolve p-toluenesulfonyl chloride (0.953g, 5mmol) in 20mL of dichloromethane and drop it into the reaction system within 2 hours using a constant velocity funnel , overnight reaction at room temperature. After the reaction, dichloromethane was removed by rotary evaporation under reduced pressure, and purified by silica gel column to obtain the final product Compound 3 with a yield of 79%.
[0089] (2) Synthesis of compound ER-ZS
[0090]
[0091] Compound S-1 (100mg, 0.13mmol) and 2-(7-azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (99mg, 0.26mmol) Dissolve in 5mL N,N-dimethylformamide, then add N,N-diisopropylethylamine (48μL, 0.26mM), stir at room temperature for 1 hour, then add compound 3 (21μL, 0.14mmol) and React at room temperature for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com