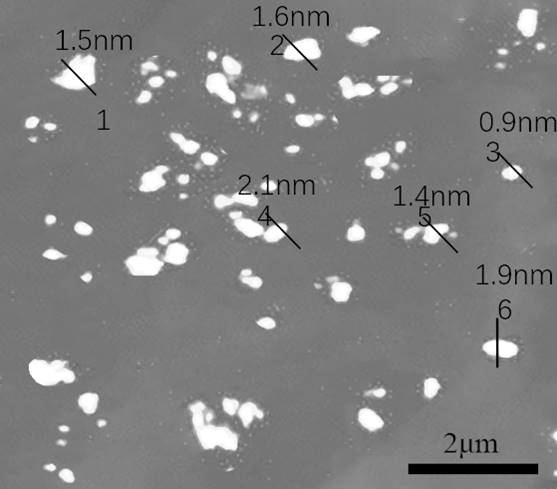
Application of nitrogen-poor g-C3N4 loaded Mg3N2 composite material as negative electrode material and lithium-based battery
A technology of composite materials and negative electrode materials, applied in the field of lithium-based batteries, can solve the problems of unstable cycle performance, insufficient electrical conductivity, weakened lithophilicity, etc., and achieve the effects of increasing nitrogen content, simple preparation method, and improved cycle stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] This embodiment provides a nitrogen-poor g-C 3 N 4 load Mg 3 N 2 Composite material (g-C 3 N 4-x / Mg 3 N 2 , where, 0x <4), the steps include:
[0030] Step 1: Weigh a certain amount of urea into the corundum ark, and then place it in a muffle furnace for calcination at a temperature of 500°C to 700°C; in this example, calcine at 550°C for 30 minutes to obtain g-C 3 N 4 ;
[0031] Step 2: Weigh the g-C obtained in step 1 3 N 4 Mix evenly with magnesium powder at a ratio of 2:1 to 1:3 to obtain a precursor, by adjusting g-C 3 N 4 With the ratio of magnesium powder, different Mg can be obtained 3 N 2 The composite material of content; In this embodiment, g-C 3 N 4 Mix with magnesium powder in a mixer for 20-60 minutes according to the ratio of 2:1, 1:1 and 1:3 to obtain the precursor. Among them, the average particle size of magnesium powder is preferably between 500-1000nm to achieve uniform mix;
[0032] Step 3: Put the precursor obtained in step 2 in a ...
Embodiment 2
[0039] This embodiment provides a kind of g-C of the present invention 3 N 4-x / Mg 3 N 2 Composite material is used as the negative electrode sheet of negative electrode material, and its preparation method comprises:
[0040] g-C of the present invention 3 N 4-x / Mg 3 N 2 Uniformly mix with conductive agent, binder and nitrogen methyl pyrrolidone to obtain slurry; pull the slurry on the current collector by scraping, and keep the temperature at 60~100°C for 6~12h . After cooling, pole pieces are obtained.
[0041] In this example, weigh a certain amount of g-C obtained in Example 1 3 N 4 load Mg 3 N 2 For the composite material, mix it with super-P and PVDF (polyvinylidene fluoride) according to the mass ratio of 8:1:1, and use NMP (nitromethylpyrrolidone) as the solvent to uniformly mix it into a slurry; use a scraper on the copper foil After drawing the film on top, put it in a vacuum oven at 80°C for drying; after cooling, cut the pole piece into a disk pole p...
Embodiment 3
[0043] The present embodiment provides the nitrogen-poor g-C containing the present invention 3 N 4 load Mg 3 N 2 Composite batteries, and testing the nitrogen-depleted g-C of the present invention 3 N 4 load Mg 3 N 2 Electrochemical performance of composite materials as anode materials. The specific implementation is as follows: assemble the 6mm disc pole piece obtained in Example 2 and the metal lithium piece into a 2032 button battery, conduct a constant current charge and discharge test, observe the cycle stability, the pure copper foil electrode and the g-C 3 N 4 electrode as a comparative example.
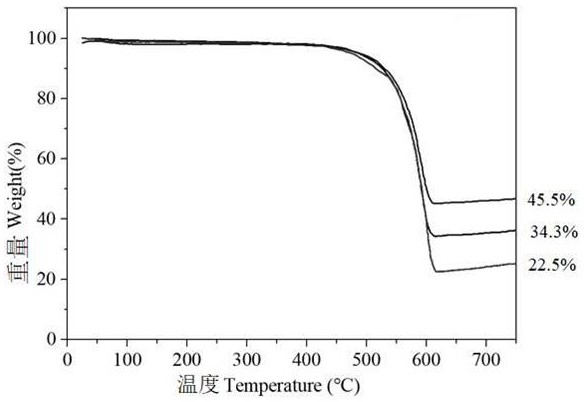
[0044] Figure 7 Different contents of g-C are given 3 N 4-x / Mg 3 N 2 Lithium metal deposition / extraction coulombic efficiency diagram, it can be seen that g-C 3 N 4-x / Mg 3 N 2 -34.3 showed the best cycle stability, after 700 cycles, the Coulombic efficiency is still close to 100%, g-C 3 N 4-x / Mg 3 N 2 -22.5 The battery fails at around 450 cycles, whil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Sheet thickness | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com