Application of epalrestat in preparation of medicine for preventing and treating cerebral arterial thrombosis
An ischemic stroke and epalrestat technology, applied in the field of medicine, can solve the problem of lack of prevention and treatment drugs for ischemic brain injury, and achieve the effects of reducing endothelial cell apoptosis, reducing infiltration, and reducing inflammatory response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
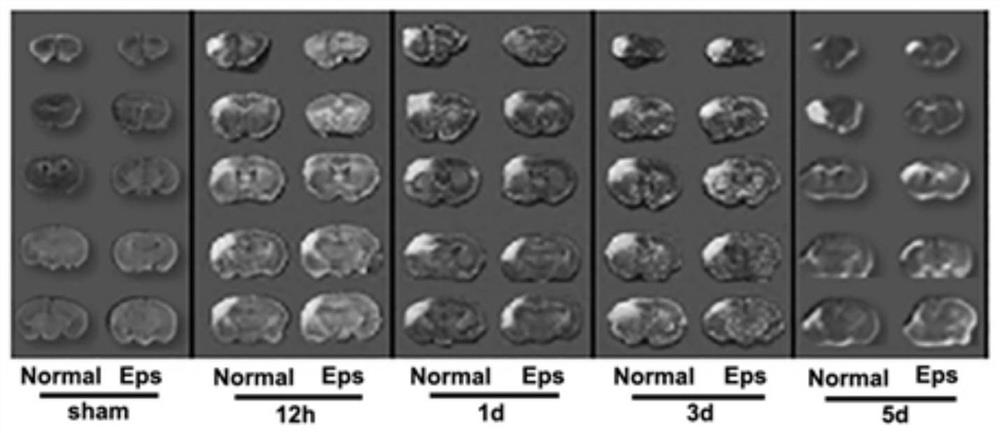
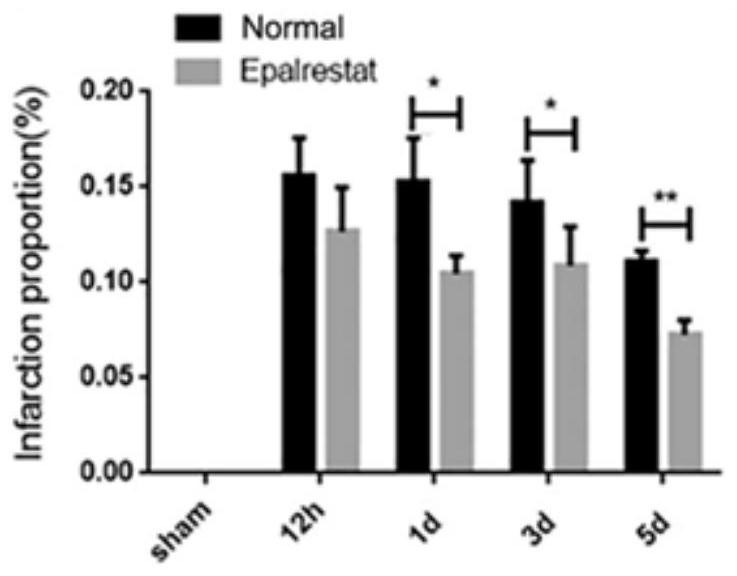
[0055] This example investigates the effect of epalrestat on cerebral ischemic injury in the process of cerebral ischemia.
[0056] The experimental animals used in the present invention are all purebred C57BL / 6 male mice with a body weight of 19-21 g, purchased from Liaoning Changsheng Biotechnology Co., Ltd.
[0057] Modeling, grouping, and administration methods: establish the mouse permanent middle cerebral artery occlusion model (pMCAL) and the sham operation group sham, respectively, and divide the pMCAL model and the sham operation (sham) group into normal (ischemic) groups - labeling The normal group, the epalrestat treatment group-Eps group and the sham group were intragastrically administered immediately after the establishment of the model. The dosage of the Eps group was 100 μg / g, and the Normal group was intragastrically administered with the same amount of normal saline.
[0058] Brain tissue sections were taken at the time points of ischemia time of 12h, 1d, 3d ...
Embodiment 2
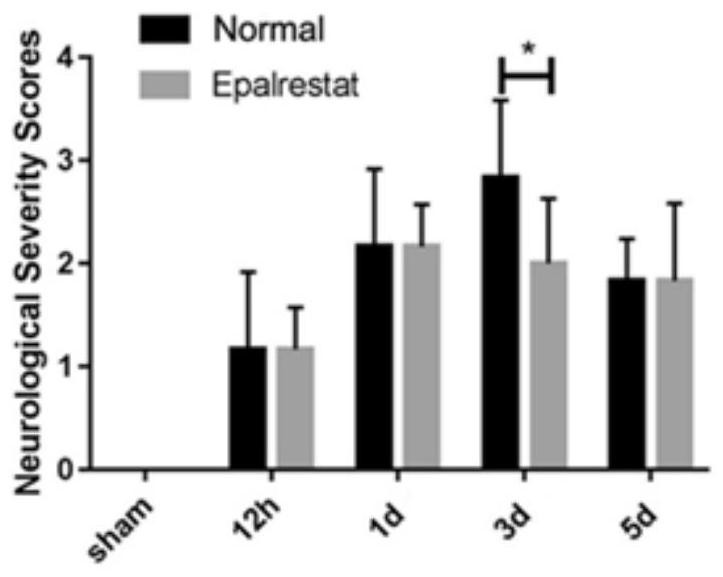
[0061] This example investigates the effect of epalrestat on the neurobehavioral function of mice during cerebral ischemia.
[0062] This example observes the state and neurobehavioral changes of mice after cerebral ischemia, and reflects the severity of cerebral ischemia in mice. According to the experimental plan, mice are modeled with pMCAL, and the modeling, grouping, and administration methods are the same as in Example 1. same. The neurological score of normal mice is 0, and the more severe the symptoms related to ischemia, the greater the score. The neurobehavioral scoring criteria are shown in Table 1:
[0063] Table 1
[0064] Neurological Svevrity Scores neurobehavioral score Normal neurological function 0 marks Mild neurological deficit (flexion of right forelimb when tail is raised) 1 point Moderate neurological deficit (circles to the right while walking) 2 minutes Moderate neurological deficit (slope to the right) 3 points...
Embodiment 3
[0067] This example investigates the effect of epalrestat on the blood-brain barrier function during cerebral ischemia.
[0068] The modeling, grouping, and administration methods of this embodiment are all the same as in Example 1, and the mice are injected with Evans Blue (EB) dye into the tail vein at the time point when the ischemia time is 12h, 1d, 3d, and 5d, and after 2h The brain tissue was removed by perfusion to observe the penetration of the blue dye in the brain tissue. The result is as Figure 4 As shown, the blue is the damaged area of the blood-brain barrier, and the white is the normal brain tissue; cerebral ischemia damage leads to the destruction of the integrity of the blood-brain barrier, and the EB dye can enter the brain parenchyma through the damaged blood-brain barrier to stain the ischemic area in blue .
[0069] Through trichloroacetic acid extraction, the content of EB in the brain tissue of each group was detected by ultraviolet spectrophotomete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com