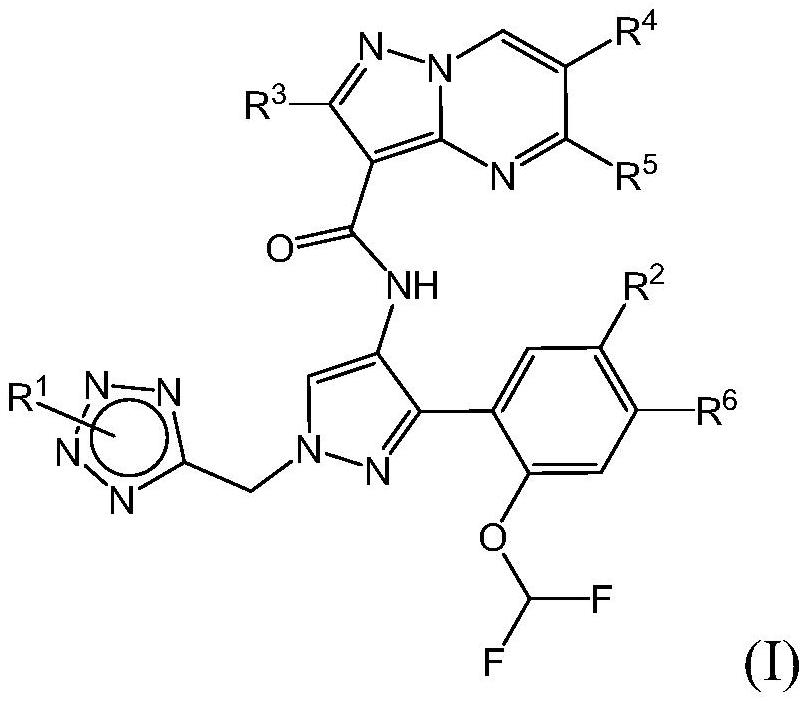
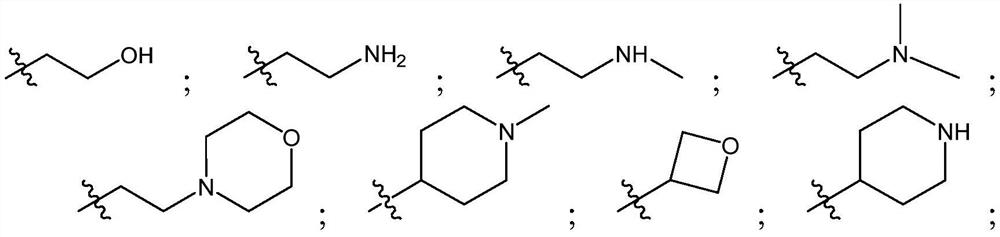
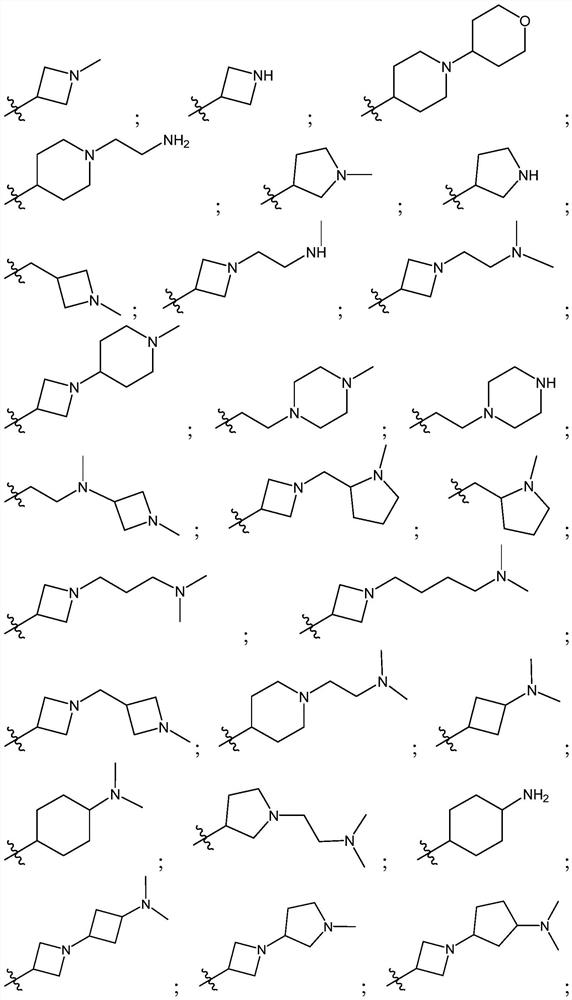
Tetrazole-substituted pyrazolopyrimidine inhibitors of jak kinases and uses thereof
A medicinal salt and pyrrolidinyl technology, which is applied in tetrazole-substituted pyrazolopyrimidine JAK kinase inhibitors and its application field, can solve problems such as defective signal transduction, achieve the requirement of reducing the frequency of administration, dissolve Sex-enhancing, dose-reducing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0350] The following representative compounds of Table 1 were prepared using procedures similar to those described in the Schemes and Examples herein. The absolute stereochemistry of each of the following compounds may not have been drawn: thus, structures may occur more than once, each representing a single stereoisomer. Table 1 also shows the LC-MS method, retention time and m / z.
[0351] Table 1: Exemplary JAK Inhibitors
[0352]
[0353]
[0354]
[0355]
[0356]
[0357]
[0358]
[0359]
[0360]
[0361]
[0362]
[0363]
[0364]
[0365]
[0366]
[0367]
[0368]
[0369] Table 1
[0370] General Experiment Details
[0371] All solvents and commercially available reagents were used as received unless otherwise stated. In the case of product purification by silica gel chromatography, a glass column manually packed with silica gel (Kieselgel 60, 220-440 mesh, 35-75 μm) was used or A SPE Si II column was used for ...
example 1
[0686]
[0687] N-(3-(5-chloro-2-(difluoromethoxy)phenyl)-1-((2-(2-hydroxyethyl)-2H-tetrazol-5-yl)methyl)- 1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
[0688] N-[3-[5-chloro-2-(difluoromethoxy)phenyl]-1-(2H-1,2,3,4-tetrazol-5-ylmethyl)-1H-pyridine Azol-4-yl]pyrazolo[1,5-a]pyrimidine-3-carboxamide (Intermediate 12, 200mg, 0.412mmol), 1,3-dioxolan-2-one (122mg, 1.38mmol , 3.35 equivalents), sodium hydroxide (44 mg, 1.10 mmol, 2.67 equivalents), N,N-dimethylformamide (30 mL) were placed in a 100 mL round bottom flask. The resulting solution was stirred in an oil bath at 120 °C for 4 h. The resulting mixture was concentrated under vacuum. The residue was applied on a silica gel column using dichloromethane / petroleum ether (11.5:1). The crude product was purified under the following conditions by chiral Prep-HPLC (Prep-HPLC-009): column, Phenomenex Lux 5u Cellulose-4XIAPacked, 2.12*25cm, 5um; mobile phase, Hex and ethanol (60.0% ethanol was maintained within 32m...
example 21
[0690] N-(3-(2,5-bis(difluoromethoxy)phenyl)-1-((2-(1-(2-(dimethylamino)ethyl)azetidine-3 -yl)-2H-tetrazol-5-yl)methyl)-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
[0691]
[0692] Step 1: 3-(5-((3-(2,5-bis(difluoromethoxy)phenyl)-4-(pyrazolo[1,5-a]pyrimidine-3-carboxamido) Synthesis of tert-butyl -1H-pyrazol-1-yl)methyl)-2H-tetrazol-2-yl)azetidine-1-carboxylate
[0693]
[0694] 1-Boc-3-iodoazetidine (2.42 g, 8.55 mmol) was added to N-[3-[2,5-bis(difluoromethoxy)phenyl]-1-(2H-tetra Azol-5-ylmethyl)pyrazol-4-yl]pyrazolo[1,5-a]pyrimidine-3-carboxamide (Intermediate 1, 1.87g, 3.61mmol) and potassium carbonate (2.43g, 17.6 mmol) in a mixture in N,N-dimethylformamide (20 mL). The resulting solution was stirred at 60 °C for 3 h and at 75 °C overnight. The mixture was cooled to room temperature and filter. The filtrate was diluted with brine (120 mL). The resulting mixture was extracted with EA (3x50 mL) and the organic layers were combined. The organic l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com