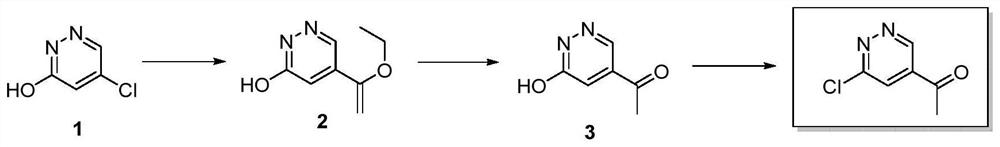
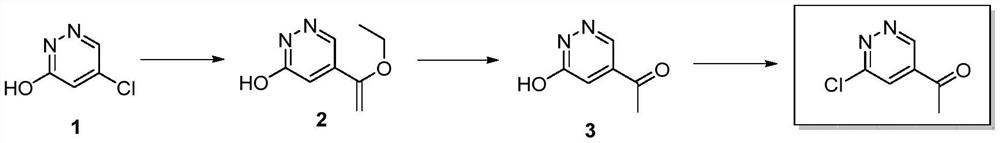
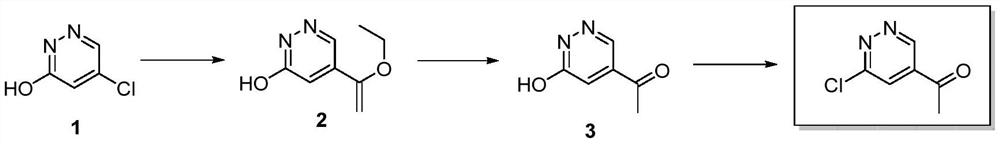
Preparation method of 1-(6-chloropyridazine-4-yl)ethyl-1-one
A technology of chloropyridazine and ethyl, applied in the field of preparation of pharmaceutical intermediates, can solve problems such as unreported synthesis methods, and achieve the effects of simple post-processing, good stability and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method of 1-(6-chloropyridazin-4-yl)ethyl-1-one, comprising the steps:
[0028] (1) DMF (50 mL, 0.65 mol) was added to a 100 mL single-necked flask, compound 1 (5-chloropyridazin-3-ol, 10.0 g, 76.92 mmol, 1 eq) was added under stirring, and the solution was clarified after stirring for 5 minutes. Tributyl (1-ethoxyethylene) tin (27.8g, 76.92mmol, 1eq), triethylamine (15.5g, 153.84mmol, 2eq) were successively added to it, and bistriphenylphosphonium disulfide was added under nitrogen protection Palladium chloride (2.7g, 3.85mmol, 0.05eq), then continued to react at 90°C for 3h under nitrogen protection, cooled to room temperature, added water to quench the reaction, and then extracted with ethyl acetate 3 times, the organic phases were combined, Dry over anhydrous sodium sulfate, filter, and concentrate to obtain the crude product. The crude product is purified by silica gel column chromatography and eluted with dichloromethane:methanol=20:1 volume ratio is...
Embodiment 2
[0036] A preparation method of 1-(6-chloropyridazin-4-yl)ethyl-1-one, comprising the steps:
[0037] (1) DMF (50 mL, 0.65 mol) was added to a 100 mL single-necked flask, compound 1 (5-chloropyridazin-3-ol, 10.0 g, 76.92 mmol, 1 eq) was added under stirring, and the solution was clarified after stirring for 5 minutes. Tributyl(1-ethoxyethylene)tin (41.76g, 115.38mmol, 1.5eq), DIEA (29.7g, 230.76mmol, 3eq) were successively added thereto, and bistriphenylphosphorus dichloride was added under nitrogen protection. Palladium (5.4 g, 7.70 mmol, 0.1 eq), then continued to react at 120 ° C for 1 h under nitrogen protection, cooled to room temperature, added water to quench the reaction, and then extracted with ethyl acetate 3 times, the organic phases were combined, no Dry over sodium sulfate, filter, and concentrate to obtain the crude product. The crude product is purified by silica gel column chromatography and eluted with dichloromethane:methanol=20:1 volume ratio isocratic to obt...
Embodiment 3
[0046] A preparation method of 1-(6-chloropyridazin-4-yl)ethyl-1-one, comprising the steps:
[0047] (1) DMF (50 mL, 0.65 mol) was added to a 100 mL single-necked flask, the raw material compound 1 (5-chloropyridazin-3-ol 10.0 g, 76.92 mmol, 1 eq) was added under stirring, and the solution was clarified after stirring for 5 minutes. Tributyl (1-ethoxyethylene) tin (27.8g, 76.92mmol, 1eq), triethylamine (38.7g, 384.60mmol, 5eq) were successively added to it, and bistriphenylphosphonium disulfide was added under nitrogen protection Palladium chloride (10.8 g, 15.4 mmol, 0.2 eq), then continued to react at 140 ° C for 1 h under nitrogen protection, cooled to room temperature, added water to quench the reaction, and then extracted with ethyl acetate 3 times, the organic phases were combined, Dry over anhydrous sodium sulfate, filter, and concentrate to obtain the crude product. The crude product is purified by silica gel column chromatography and eluted with dichloromethane:methan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com