High-thermal-conductivity hydrated nitrate composite phase change material and preparation method thereof
A composite phase change material and high thermal conductivity technology, applied in the field of high thermal conductivity hydrated nitrate composite phase change material and its preparation, can solve the problems of composite phase change material leakage and weak adsorption capacity of hydrated salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The modified expanded graphite process is carried out in the following manner:
[0057] First, about 2 g of expandable graphite was weighed into a crucible, and the crucible was transferred to a muffle furnace at 500 °C and kept for 10 min. After the expansion process is completed, the muffle furnace is naturally lowered to room temperature, and the resulting product is collected, which is expanded graphite (EG). Next, soak the obtained EG in 20% absolute ethanol solution, add 0.2mol L -1 A certain volume of Al(NO 3 ) 3 9H 2 O solution, then the resulting solution was adjusted to neutral with ammonia water, and stirred at room temperature for 1 h. The fully reacted mixed solution was filtered, and the solid was dried at 60 °C for 24 h. Finally, put it into a muffle furnace at 500°C for calcination for 3 hours to obtain the modified expanded graphite MEG with a layer of aluminum oxide film on the surface.
[0058] Add 0.2mol L -1 Al(NO 3 ) 3 9H 2 When the O sol...
Embodiment 2
[0068] The preparation of the hydrated nitrate composite phase change material with high thermal conductivity is carried out in the following manner:
[0069] The MEG (N5) obtained in Example 1 is used as the carrier material, and calcium nitrate tetrahydrate (CN-4W) is placed in a water bath for heating and melting. Then, MEG with a mass ratio of 0, 5wt%, 8wt% and 10wt% (the mass percentage is calculated according to the total mass of the composite phase change material obtained) is mixed with the molten CN-4W respectively, uniformly adsorbed, and obtained after cooling. phase change material. Denote as CN-4W (1), 5 wt% MEG+CN-4W (code 2), 8 wt% MEG+CN-4W (code 3) and 10 wt% MEG+CN-4W (code 4).
[0070] Figure 5 It is the photos of four groups of calcium nitrate tetrahydrate (CN-4W) composite phase change materials after the operation of the tablet machine.
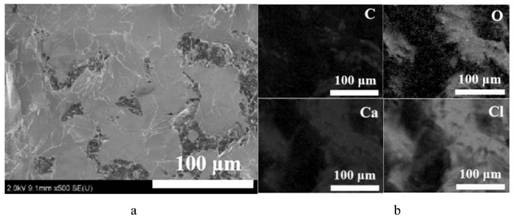
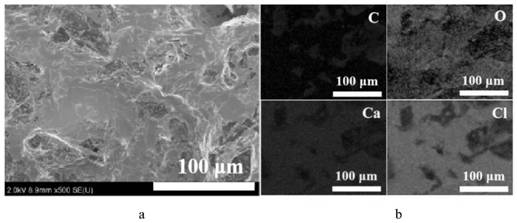
[0071] Such as Figure 6 as shown in a. It can be observed from the XRD patterns of CN-4W and composite phase ch...
Embodiment 3
[0080] High thermal conductivity CaCl 2 ·6H 2 O-Ca(NO 3 ) 2 4H 2 O composite phase change energy storage material, adopt the following method to carry out:
[0081] With the MEG (N5) that arrives in embodiment 1 as carrier material, be that the calcium chloride hexahydrate of 93% and the calcium nitrate tetrahydrate that mass percent is 7% with mass percent are the mixed phase change inorganic salt (937) that forms as The phase change inorganic hydrated salt is heated and melted in a water bath. Then, MEG with a total mass percentage of 0wt%, 5wt%, 8wt% and 10wt% is mixed and stirred with molten mixed phase-change inorganic salt (937), uniformly adsorbed, and the composite phase-change material is obtained after cooling. The prepared composite phase change materials are respectively marked as 937, 5wt% MEG+937, 8wt% MEG+937 and 10wt% MEG+937.
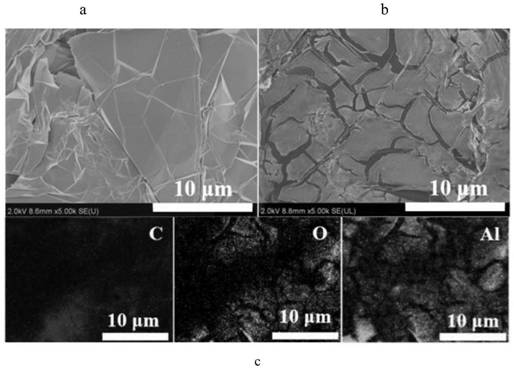
[0082] Figure 12-1 (a) for EG and Figure 12-1 (b) SEM image of MEG. It can be seen from the figure that Al is used on the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com