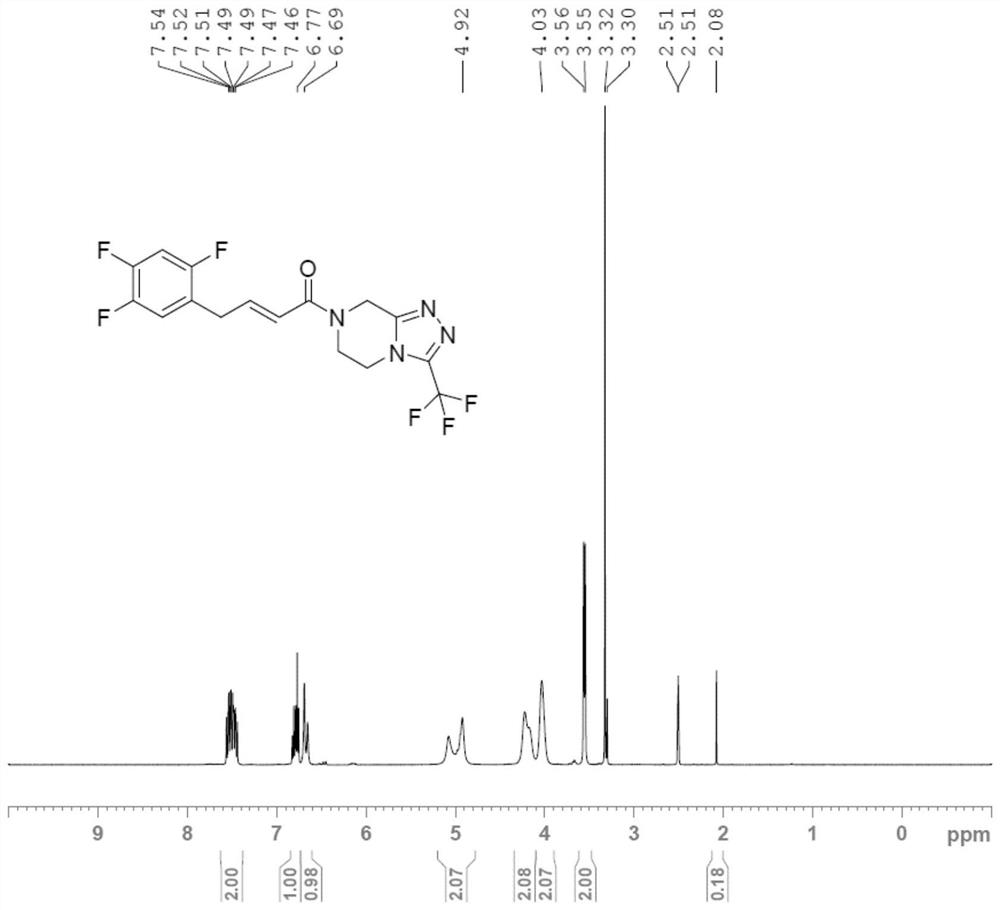
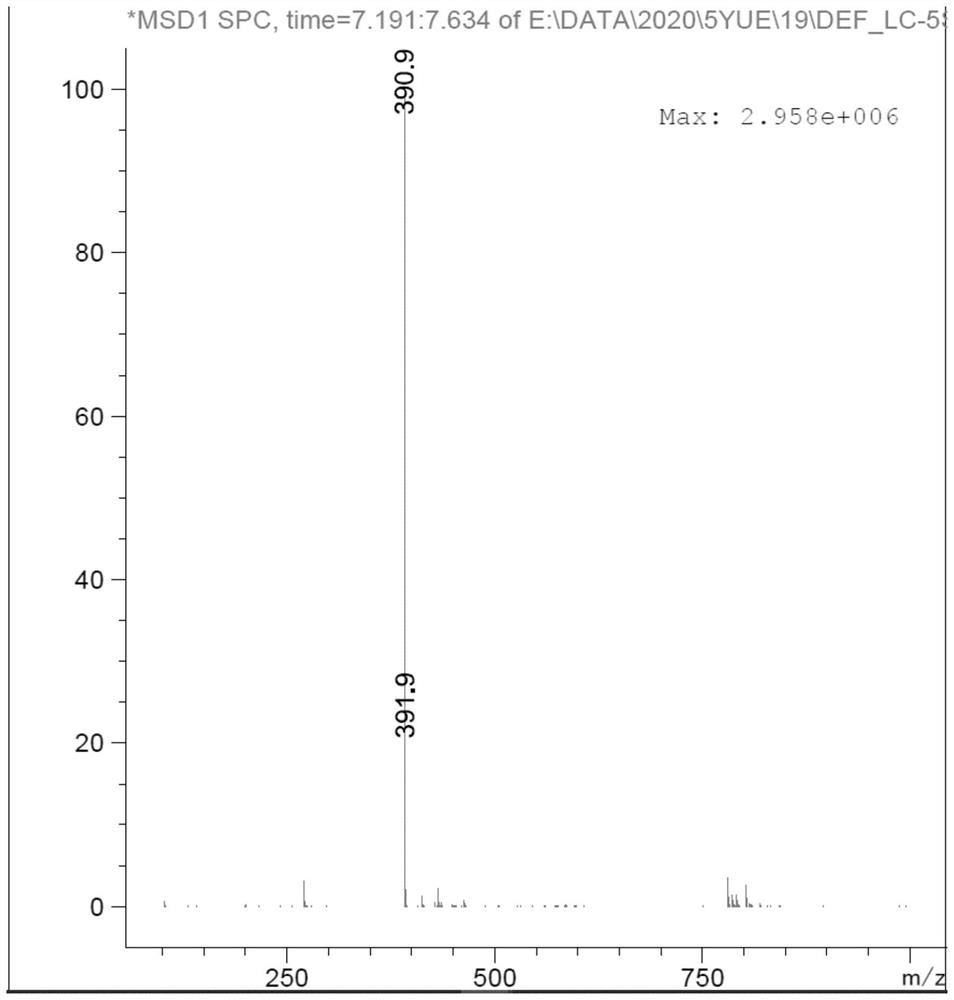
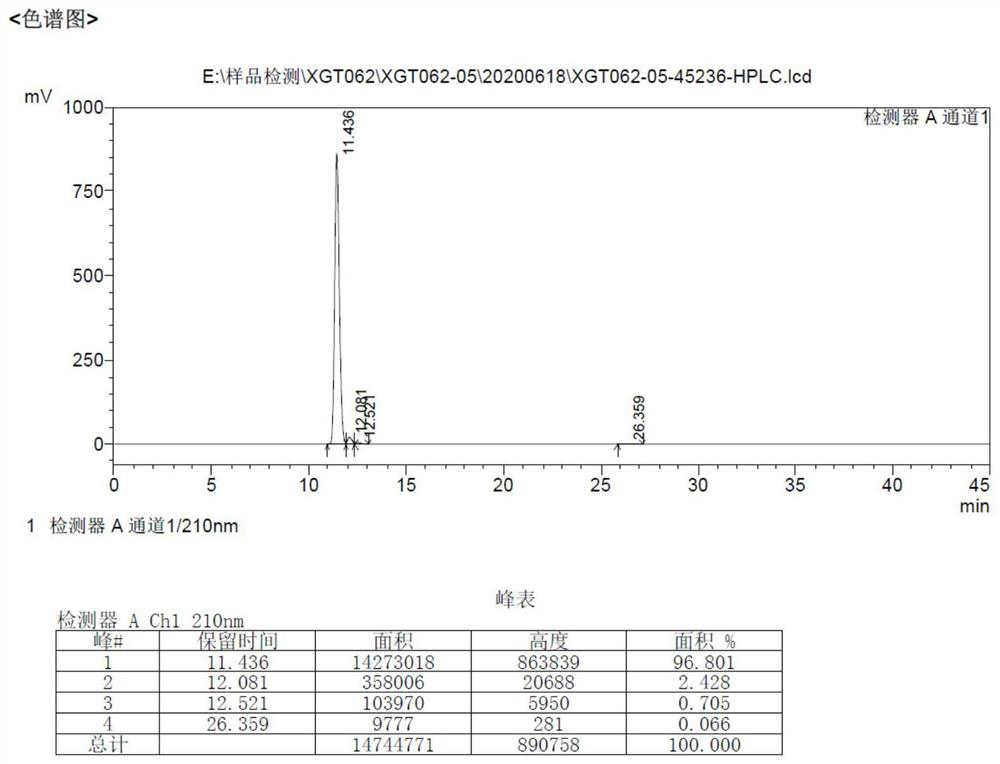
Preparation method of sitagliptin impurity
A sitagliptin impurity and quality technology, which is applied in the field of sitagliptin impurity preparation, can solve the problems of difficulty in repetition, cumbersome operation, increased purification cost, etc., and achieves the effects of rigorous and realistic operation, easy availability of materials, and convenient purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The synthesis of embodiment 1 compound XGT062-05-01
[0047]
[0048] Add XGT062-05-001 (30g, 1.0eq), formaldehyde (23.8g, 4.0eq), methanol (300mL), sodium cyanoborocyanide (18.45g, 4.0eq) into a 500mL three-necked flask, and replace with argon Three times, stirred overnight at room temperature, and monitored the progress of the reaction by TLC. The basic reaction of raw materials is completed, stop the reaction, add water to stir, extract with DCM for 3 times, combine the organic phases, wash with saturated sodium chloride, and shrink to dry to obtain a yellow oily crude product, which is purified by column chromatography, the eluent volume ratio DCM / MeOH=20 / 1 , collected the eluate and shrunk to dry to obtain 16.4 g of light yellow oily product. Yield: 52%.
Embodiment 2
[0049] The synthesis of embodiment 2 compound XGT062-05
[0050]
[0051] Take the compound XGT062-05-01 (16.4g, 1.0eq) obtained in step 1, dissolve it in dichloromethane (165mL) and add it to a three-neck flask, cool down and stir, when the temperature is 0-5°C, add m-chloroperoxygen dropwise A solution of benzoic acid (11.6g, 1.5eq) in DCM was added after dropping, and the reaction was incubated for 1 h, and the progress of the reaction was monitored by TLC. After the reaction of the raw materials was complete, a saturated aqueous solution of sodium bicarbonate was added to stir, and the liquids were separated. The organic phase was washed 3 times with an aqueous solution of sodium sulfite to remove m-chloroperoxybenzoic acid. The organic phase was dried and concentrated to obtain 17 g of a light yellow oily crude product. Purify the crude product by column chromatography, eluent: DCM:MeOH=40:1, collect the eluate, and concentrate to dryness under reduced pressure. The ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com