Method for preparing cyclohexanone by cyclohexane oxidation
A technology of cyclohexanone and cyclohexane, applied in the field of preparation of cyclohexanone, can solve the problems of low two-phase mass transfer efficiency, many by-products, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
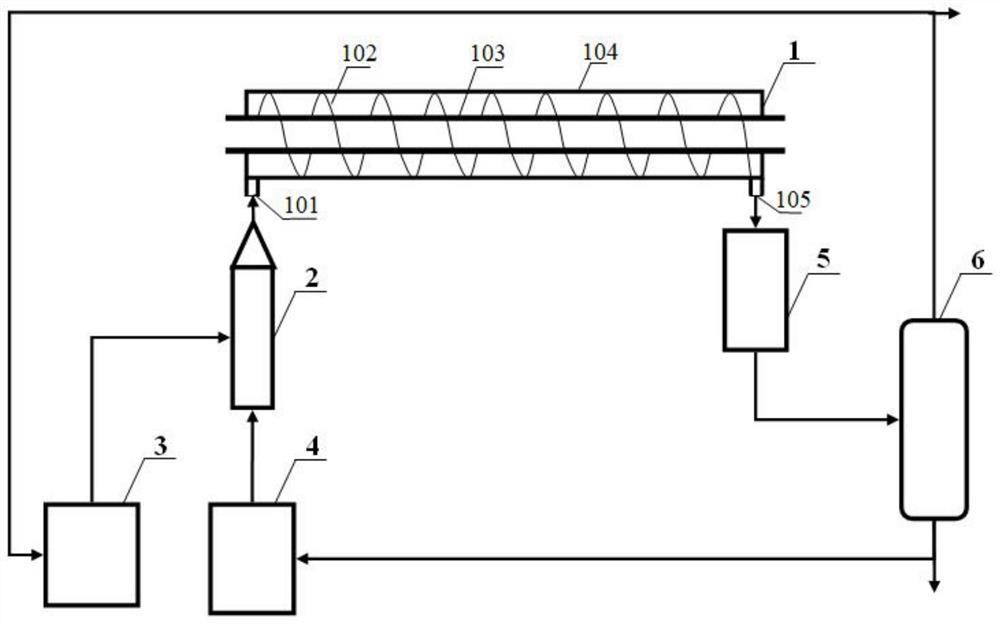
[0024] see figure 1 , the present invention provides a technical solution, a method for producing cyclohexanone by oxidation of cyclohexane, comprising a device for producing cyclohexanone by oxidation of cyclohexane, and the device for producing cyclohexanone by oxidation of cyclohexane includes a tubular reactor 1. Ultrasonic microbubble generator 2, air storage tank 3, cyclohexane storage tank 4, condenser 5 and gas-liquid separator 6, characterized in that the preparation method comprises the following steps:
[0025] Step 1: The air storage tank 3 and the cyclohexane storage tank 4 are fed in a certain proportion, and the mixing is strengthened by the ultrasonic microbubble generator 2, so that the gas is broken into microbubbles and enters the tubular reactor 1. After the reaction, the materials pass through the condenser 5 cooling, gas-liquid separator 6 separation to obtain cyclohexane oxidation liquid;
[0026] Step 2: catalytically decompose the cyclohexane oxidatio...
Embodiment 1
[0028] The spiral dividing plate (102) in the tubular reactor (1) forms an included angle of 45° with the reactor axis, and the reaction temperature in the tubular reactor (1) is controlled by the heat exchange tube (103) to be 130°C. The pressure is 1.5MPa, the air feed rate is controlled to make the reaction gas-liquid separation, and the oxygen volume content in the gas phase is 1.2%, and the diameter of the microbubbles entering the tubular reactor (1) is 0.3mm. The chromium ion concentration of the ester catalyst is 8ppm, and under the conditions of a reaction temperature of 100°C, it decomposes slightly acidicly for 30 minutes, and then decomposes alkalinely for 20 minutes.
[0029] Cyclohexane oxidation per pass conversion rate 7.9%, selectivity of cyclohexanol, cyclohexanone and cyclohexyl hydroperoxide 93%, decomposition rate of cyclohexyl hydroperoxide 99.8%, total yield of cyclohexanone and cyclohexanol 99.5% , The ratio of ketone to alcohol is 1.4:1.
Embodiment 2
[0031] The spiral partition plate 102 in the tubular reactor 1 forms an included angle of 75° with the reactor axis, and the reaction temperature in the tubular reactor 1 is controlled by the heat exchange tube 103 to be 160°C, the reaction pressure is 0.5MPa, and the air inlet is controlled. The volume content of oxygen in the gas phase is 1.0% after the reaction gas-liquid separation, the diameter of the microbubbles entering the tubular reactor 1 is 0.1mm, and the chromium ion concentration of the oil-soluble isooctyl chromate catalyst is 2ppm, and the reaction temperature Under the condition of 130°C, first decompose slightly acidic for 60 minutes, and then decompose alkalinely for 60 minutes.
[0032] Cyclohexane oxidation per pass conversion rate 7.3%, selectivity of cyclohexanol, cyclohexanone and cyclohexyl hydroperoxide 95%, decomposition rate of cyclohexyl hydroperoxide 99.7%, total yield of cyclohexanone and cyclohexanol 99.5% , The ratio of ketone to alcohol is 1.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com