Cyclic beta-1,2-glucan and curcumin inclusion compound and preparation method thereof
A technology of dextran and curcumin, applied in the biological field, can solve the problems of low water solubility, low bioavailability, and poor stability of curcumin, and achieve the effect of low bioavailability, simple preparation process, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1: Preparation of cyclic β-1,2-glucan
[0093] Specific steps are as follows:
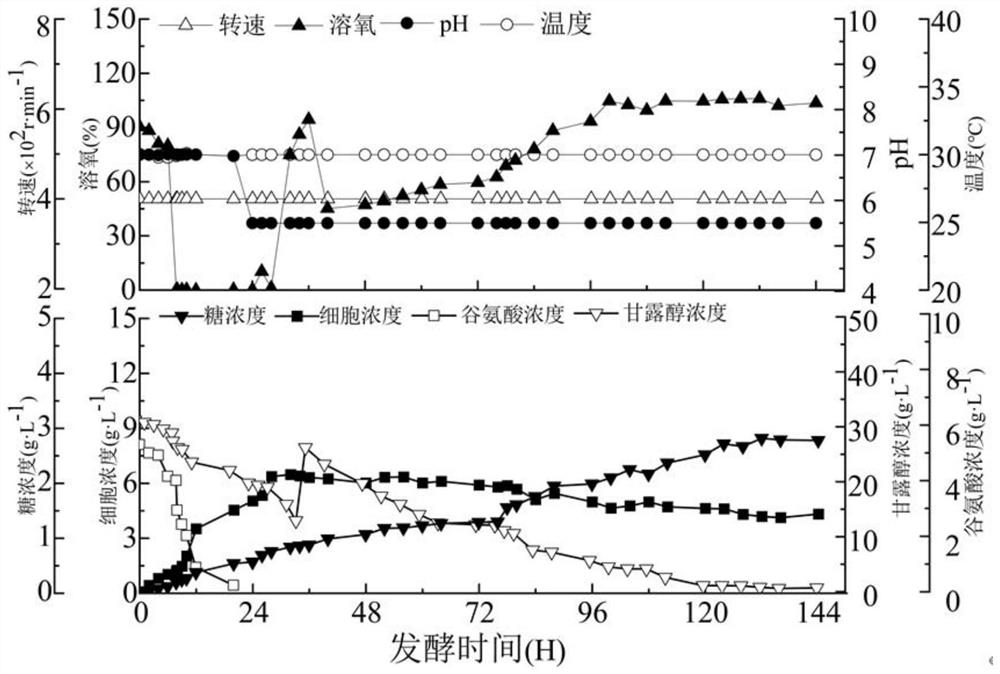
[0094] 1. Preparation of extracellular neutral oligosaccharides by shake flask fermentation of Rhizobium radiobacter ATCC 1333
[0095] (1) Inoculate Rhizobium radiobacter ATCC 1333 on the slant medium, and culture it upside down at 30°C. After culturing for 48-72 hours, pick 2-3 rings of single colonies and inoculate them into 50mL seed medium on the ultra-clean bench , 30°C, 200rpm conditional culture for 24h, the activated seed solution is obtained;
[0096] (2) Inoculate the seed liquid obtained in step (1) in the fermentation medium with an inoculum size of 10% (v / v), 30°C, 200rpm, ferment and cultivate for 144h, prepare a fermented liquid, and detect the fermented liquid in the fermented liquid The extracellular neutral oligosaccharides (the total sugar content in the fermentation broth minus the acid sugar content, roughly estimated as the neutral sugar content), its content...
Embodiment 2
[0104] Example 2: Purification of cyclic β-1,2-glucan
[0105] Specific steps are as follows:
[0106] Step 1: Concentrate the fermentation supernatant obtained in Example 1 by rotary evaporation, centrifuge at 10,000 rpm for 10 minutes, take the supernatant, add 3 times the volume of absolute ethanol to the supernatant, and incubate at 4°C for 12 hours Afterwards, centrifuge at 10000rpm for 10min, and get the supernatant;
[0107] The supernatant was concentrated to 1 / 10 of the original volume to obtain a concentrated solution; 3 times the volume of absolute ethanol was added to the concentrated solution so that the final concentration of ethanol reached 75% (v / v) to obtain a mixture, which was After the mixture was placed at 4° C. for 12 hours, it was centrifuged at 10,000 rpm for 10 minutes, the supernatant was collected, and the supernatant was concentrated to 1 / 5 of the original volume to obtain a concentrate.
[0108] Step 2: Continue to add ten times the volume of abs...
Embodiment 3
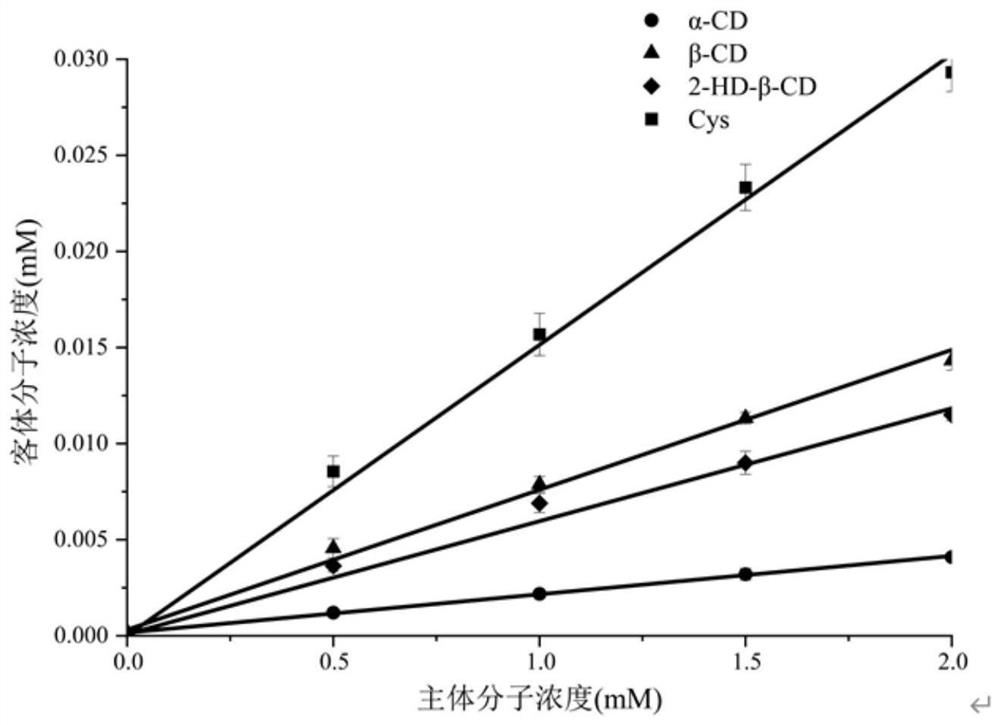
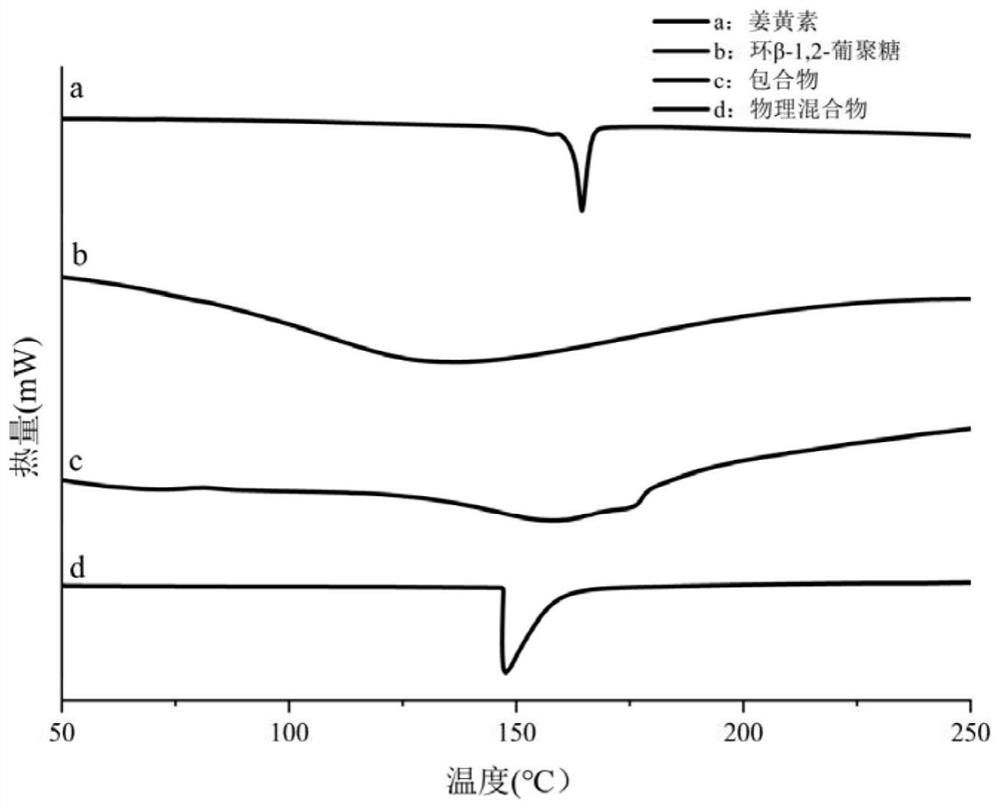
[0136] Example 3: Structural analysis of cyclic β-1,2-glucan
[0137] The neutral oligosaccharides obtained in Example 2 were subjected to structural analysis.
[0138] (1)MALDI-TOF-MS
[0139] Test conditions: The molecular weight of the purified neutral oligosaccharides was analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). The specific method is as follows: 1 μL of oligosaccharide sample (1 g / L) is spotted on the target, and dried in a desiccator under negative pressure. Spot 1 μL of DHB matrix solution on the same position, and dry in a desiccator under negative pressure. The sample is measured after mixing and crystallizing with the matrix. Analysis was performed in reflector mode in the mass range m / z 2000-4500. The results showed that the degree of polymerization of neutral oligosaccharides was 17-23.
[0140] (2) Monosaccharide composition
[0141] Test conditions: The obtained neutral oligosaccharides were co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com