Microneedle capable of controllably releasing medicine and preparation method of microneedle
A slow-release drug and microneedle technology, which is applied in the direction of drug devices, microneedles, pharmaceutical formulations, etc., can solve the problems of microneedle release and easy residue, improve practicability and flexibility of use, avoid injection pain, preparation method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
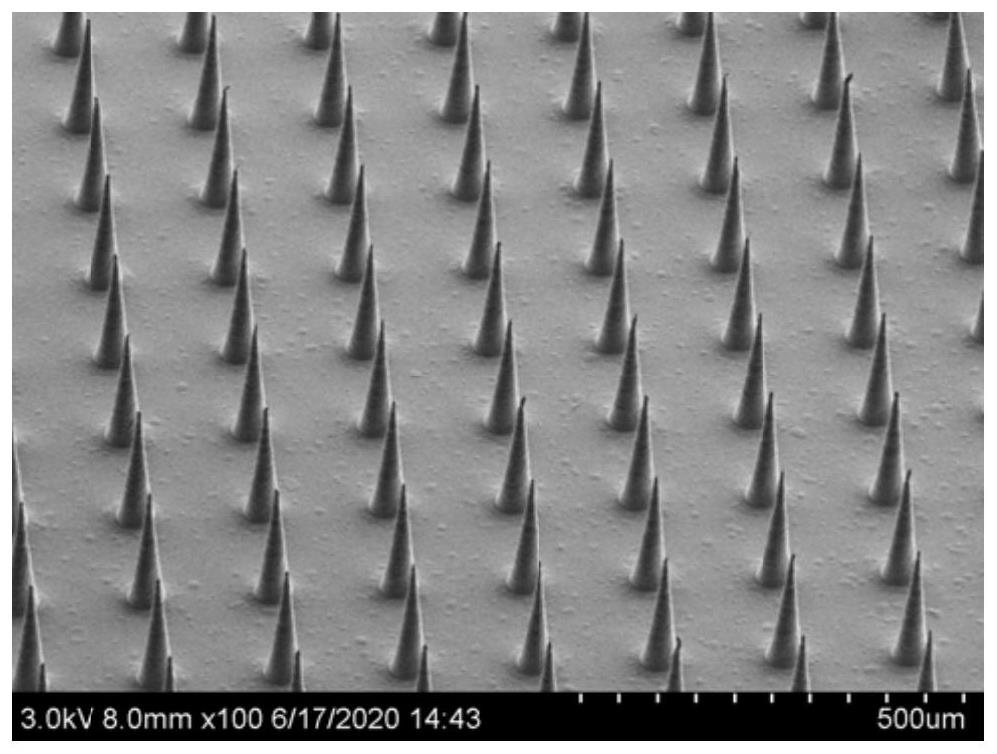
[0040] like Figure 4 , a preparation method of microneedles for controlled release of drugs, the process is as follows:
[0041] (1) Preparation of needle body fluid:
[0042] Dissolve 10 g of sodium hyaluronate (molecular weight of sodium hyaluronate: 10 kDa) in 90 g of deionized water, stir magnetically until fully dissolved, filter with a 0.22 μm filter to remove excess air bubbles, and obtain the excipient;
[0043] Dissolve 30 mg of insulin drug in 2 ml of HCl aqueous solution with a pH of 2-3, and magnetically stir until fully dissolved to obtain a drug solution;
[0044] Add the excipient liquid into the drug solution, mix well to obtain a mixed solution, slowly add NaOH aqueous solution to the mixed solution to make the pH of the mixed solution neutral, and obtain a clear and transparent needle liquid;
[0045](2) Prepare the substrate:
[0046] Weigh 0.7g of polyvinylpyrrolidone, 1.0g of sodium hyaluronate (the molecular weight of sodium hyaluronate is 130kDa) and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com