Pyrrolidine ionic liquid as well as preparation method and application thereof
A technology of ionic liquid and pyrrolidine, which is applied in the field of ionic liquid, can solve the problems of poor rate performance and poor cycle stability, and achieve the effects of high stability, improved cycle performance, and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] In addition, this embodiment also provides a method for preparing a pyrrolidine-based ionic liquid, comprising the following steps:
[0051] Step S110, according to the molar ratio of pyrrolidine and bromoalkyl ether being 1:1-1.1, dissolving pyrrolidine and bromoalkyl ether in a solvent and reacting at 50-80°C for 12-24 hours; Extraction after completion of the reaction, obtain the 1-alkoxyalkylpyrrole of following structural formula:
[0052]
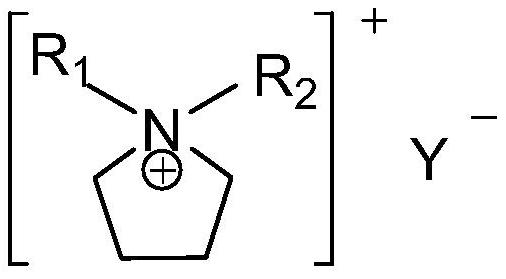
[0053] Among them, R 1 for CH 2 (CH 2 ) a O(CH 2 ) b CH 3 , a is 0 or 1, b is 0 or 1;
[0054] The resulting 1-alkoxyalkylpyrrole is subjected to vacuum distillation and fractions are collected to obtain purified 1-alkoxyalkylpyrrole
[0055] In other preferred embodiments, the molar ratio of pyrrolidine to brominated alkyl ether is preferably 1:1.05.
[0056] Step S120, under a protective gas atmosphere, according to the molar ratio of 1-alkoxyalkylpyrrole to nitrile compound is 1:1-1:1.3, dissolve 1-alkoxyalkylp...
Embodiment 1
[0078] (1) Add 0.1mol pyrrolidine, 0.105mol 2-bromoethyl methyl ether and 20mL acetonitrile respectively in a three-necked round bottom flask to obtain the mixture. Under the protection of nitrogen atmosphere, the above mixture was reacted at 50°C for 24 hours; Extract three times with 10ml of ethyl acetate, retain the organic phase solution, and carry out vacuum distillation, and collect fractions to obtain 1-methoxyethylpyrrole.
[0079]
[0080] Among them, R 1 for CH 2 (CH 2 ) a O(CH 2 ) b CH 3 , a is 1, b is 0;
[0081] (2) Add 0.075mol 1-methoxyethylpyrrole, 0.09mol bromoacetonitrile and 15ml anhydrous methanol respectively to a three-neck round bottom flask to obtain the mixture. Under the protection of nitrogen atmosphere, the above mixture is stirred and refluxed at 60°C After reacting for 14 hours, the reaction product was obtained. After the reaction was stopped, the unreacted bromoacetonitrile and solvent were removed by rotary evaporation, and the trea...
Embodiment 2
[0091] Add 0.1mol pyrrolidine, 0.105mol 2-bromoethyl ethyl ether and 20ml acetonitrile respectively to a three-neck round bottom flask to obtain a mixture. Under the protection of nitrogen atmosphere, react the above mixture at 50°C for 24 hours; Extract with ethyl acetate three times, retain the organic phase solution, and carry out vacuum distillation, and collect the fractions to obtain 1-ethoxyethylpyrrole.
[0092] Add 0.075mol 1-ethoxyethylpyrrole, 0.09mol acrylonitrile and 15ml anhydrous methanol respectively to a three-neck round bottom flask to obtain a mixture. Under the protection of nitrogen atmosphere, stir and reflux the above mixture at 60°C for 14 hours , After the reactants were obtained and the reaction was stopped, the unreacted acrylonitrile and solvent were removed by rotary evaporation, and the treated reaction product was vacuum-dried at 60°C for 24 hours to obtain a yellow transparent liquid, which was N-ethoxyethyl- N-Cytriloethylpyrrole Bromide.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com