Heterocyclic compound containing heteroatom substituted fluorene and application thereof in photoelectric device
A technology of heterocyclic compounds and heteroatoms, which is applied in the field of organic electroluminescent materials, can solve the problems that OLED devices cannot meet the market demand, the light extraction effect is not good enough, and cannot be obtained at the same time, so as to achieve improved current efficiency, excellent durability, long life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
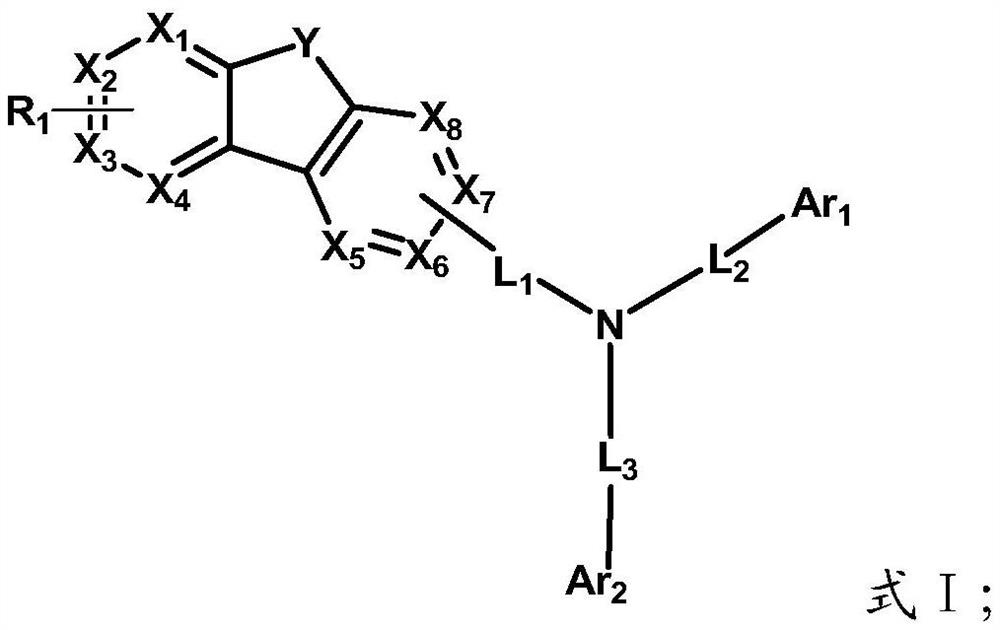
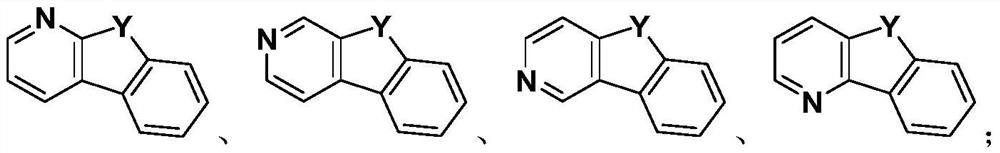
[0112] The preparation method of the above-mentioned heterocyclic compound provided by the present invention belongs to the prior art, and those skilled in the art can select a specific synthetic method according to conventional technical knowledge. The present invention only provides an exemplary synthetic route, but is not limited to the following synthetic route.
[0113] The representative synthetic route of the formula I compound provided by the invention is as follows:
[0114]
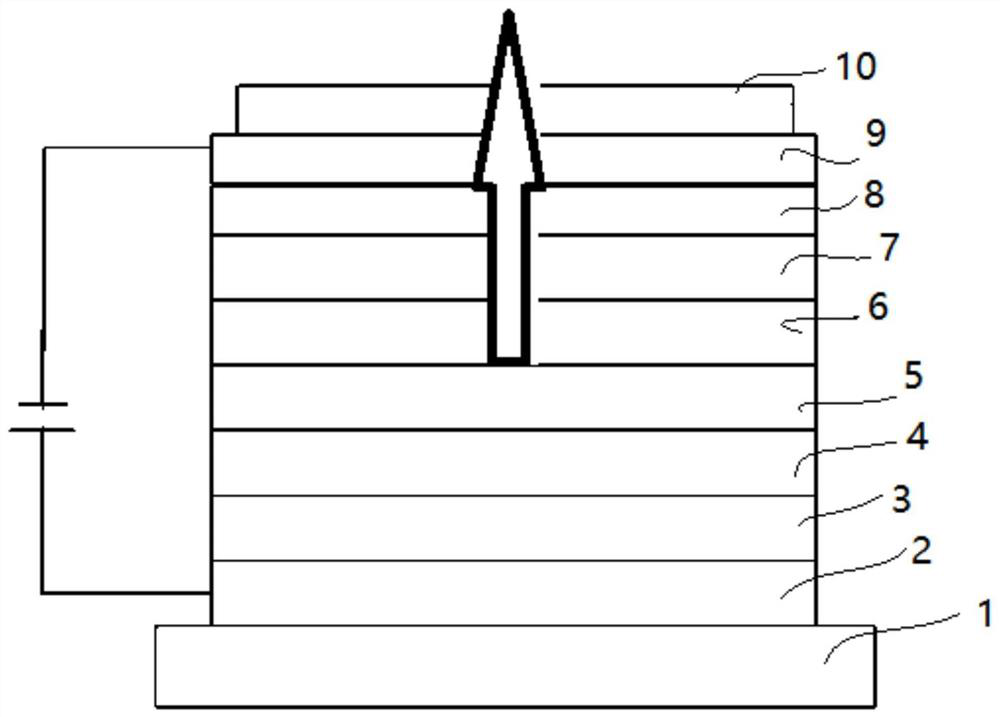
[0115] The above-mentioned compound provided by the present invention can be applied to the CPL layer of a top-emitting OLED device; it can also be used as an optical auxiliary layer such as a hole transport layer and an electron blocking layer.
[0116] The present invention provides a display panel, comprising an organic light-emitting device, the organic light-emitting device includes an anode, a cathode, and an organic thin layer between the anode and the cathode, the cathode is covered wi...
Embodiment 1
[0130] The synthetic route of compound M001 is as follows:
[0131]
[0132] Concrete preparation method comprises the following steps:
[0133]
[0134](1) M001-1 (0.5mmol), M001-2 (0.75mmol), K 2 CO 3 (0.5mmol), PdCl 2 (5×10 - 4 mmol), TPPDA (5×10 -4 mmol) was added into 3mL o-xylene solution and mixed, put into a 50mL flask, and reacted at 100°C for 24 hours. Cool to room temperature, then slowly add saturated MgSO to the solution 4 The aqueous solution and ethyl acetate were extracted three times, and then the organic layer was removed from the solvent by a rotary evaporator, and the crude product M001-3 was obtained by column chromatography.
[0135] (2) M001-3(0.5mmol), M001-4(1.5mmol), KO(t-Bu)(0.75mmol), [Pd(cinnamyl)Cl] 2 (2mol%) and Ligand (1.5mol%) were added to 3mL of toluene solution and mixed, put into a 50mL flask, and reacted at 110°C for 12 hours. Cool to room temperature, then slowly add saturated MgSO to the solution 4 The aqueous solution an...
Embodiment 2
[0139] The synthetic route of compound M029 is as follows:
[0140]
[0141] Concrete preparation method comprises the following steps:
[0142]
[0143] (1) M001-3(0.5mmol), M029-1(1.5mmol), KO(t-Bu)(0.75mmol), [Pd(cinnamyl)Cl] 2 (2mol%) and Ligand (1.5mol%) were added to 3mL of toluene solution and mixed, put into a 50mL flask, and reacted at 110°C for 12 hours. Cool to room temperature, then slowly add saturated MgSO to the solution 4 The aqueous solution and ethyl acetate were extracted three times, and then the organic layer was removed from the solvent by a rotary evaporator, and the crude product M029 was obtained by column chromatography.
[0144] Test the structure of the target product M029: MALDI-TOF MS (m / z) obtained by matrix-assisted laser desorption ionization time-of-flight mass spectrometry analysis: C 43 h 26 N 4 o 3 , the calculated value is 646.2, and the tested value is 646.3.
[0145] Elemental analysis: theoretical value C, 79.86; H, 4.05; N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com