Continuous preparation method for extracting ephedrine from ephedra
A technology for ephedrine and ephedra, which is applied in the field of natural medicine extraction, can solve the problems of long production cycle, low recovery rate, high toxicity, etc., and achieves the effects of simple operation, large processing capacity and favorable industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
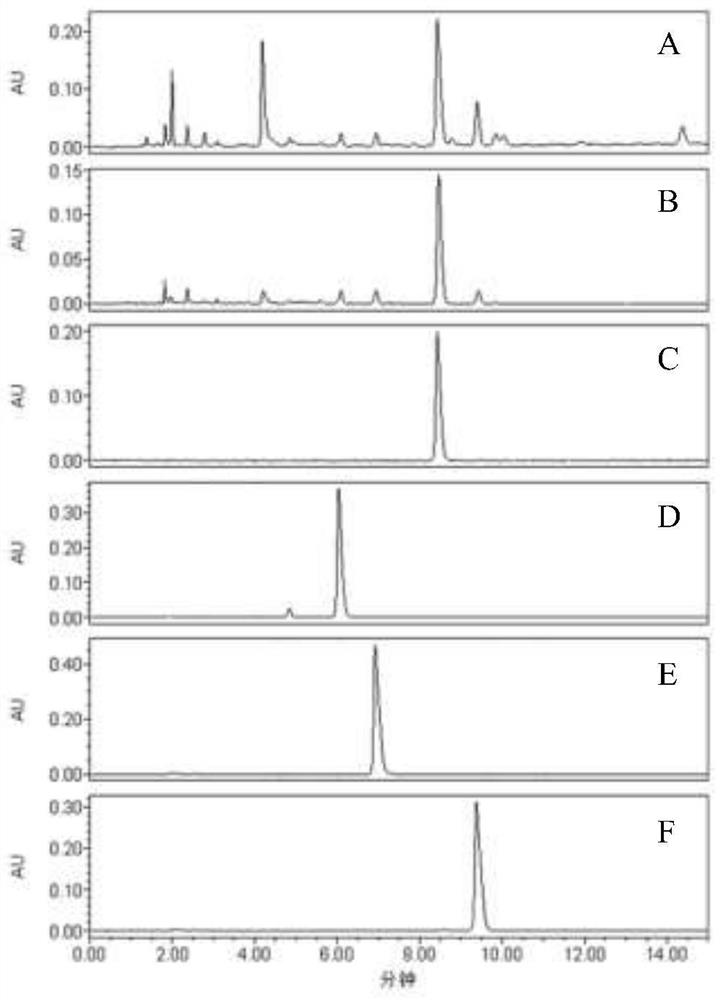
[0017] 1) Preparation of Ephedra extract: take 50g of Ephedra root and pulverize it into coarse powder, pass through a 24-mesh sieve, take the coarse powder that has passed through the sieve, and extract with 500 mL of 0.1% formic acid water (V / V) at 100°C under reflux for 1 h for a total of 1 h. Extract 2 times (repeated 1 time), obtain ephedra extract after merging the filtrate, the mass content of ephedrine in the extract is about 2.2%, the mass content of pseudoephedrine, desmethylephedrine and desmethylpseudoephedrine are successively about 0.68%, 0.14 %, 0.20%;
[0018] 2) preparation of permeate: pass the ephedra extract through 750KD hollow fiber ultrafiltration membrane to obtain permeate;
[0019] 3) Preparation of enrichment solution: After equilibrating reversed-phase column 1 (filler is C18HCE, particle size is 7 μm, inner diameter 4.6 mm, column length 250 mm) with 3 column volumes of formic acid water (final volume concentration of formic acid is 0.1%), take Th...
Embodiment 2
[0022] 1) Preparation of Ephedra extract: take 200g of Ephedra root and pulverize it into coarse powder, pass through a 60-mesh sieve, take the coarse powder under the sieve, and extract with 4000mL of 0.5% hydrochloric acid water (V / V) ultrasonic (ultrasonic power 600W, frequency 50kHz) for 1h , extract 3 times, filter to obtain ephedra extract, the mass content of ephedrine in the extract is about 1.7%, and the mass content of pseudoephedrine, desmethylephedrine and desmethylpseudoephedrine are about 0.51%, 0.10%, 0.13% successively;
[0023] 2) Preparation of permeate: the ephedra extract was passed through a 500KD hollow fiber ultrafiltration membrane to obtain permeate;
[0024] 3) Preparation of enrichment solution: After equilibrating the reversed-phase column 1 (filler is C18HCE, particle size is 10 μm, inner diameter 20 mm, column length 250 mm) with 4 times the column volume of formic acid water (the final volume concentration of formic acid is 0.5%), take the step (...
Embodiment 3
[0027] 1) Preparation of Ephedra extract: take 1000g Ephedra root and pulverize it into coarse powder, pass through a 100-mesh sieve, take out the coarse powder, and extract with 15L of 1% acetic acid water (V / V) under reflux for 1.5h, extract twice, and combine the filtrates After obtaining ephedra extract, the mass content of ephedrine in the extract is about 2.4%, and the mass contents of pseudoephedrine, demethylephedrine and demethylpseudoephedrine are about 0.65%, 0.16%, and 0.18% successively;
[0028] 2) preparation of permeate: pass the ephedra extract through 750KD hollow fiber ultrafiltration membrane to obtain permeate;
[0029] 3) Preparation of enrichment solution: reversed-phase column 1 (filler is C18HCE, particle size is 40 μm, inner diameter 50 mm, column length 250 mm) is equilibrated with 5 times the column volume of formic acid water (the final volume concentration of formic acid is 1%), and the step ( 2) The obtained permeate was applied to the above-ment...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com