Escherichia coli membrane protein ZraS mutant, gene for coding mutant, recombinant vector as well as preparation method and application of Escherichia coli membrane protein ZraS mutant
A technology of Escherichia coli and mutants, applied in the field of genetic engineering, can solve the problems of complex components of samples to be tested, difficulty in achieving detection targets in wild-type systems, inability to specifically distinguish lead ions and zinc ions, etc., achieving low cost and improved response sensitivity , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
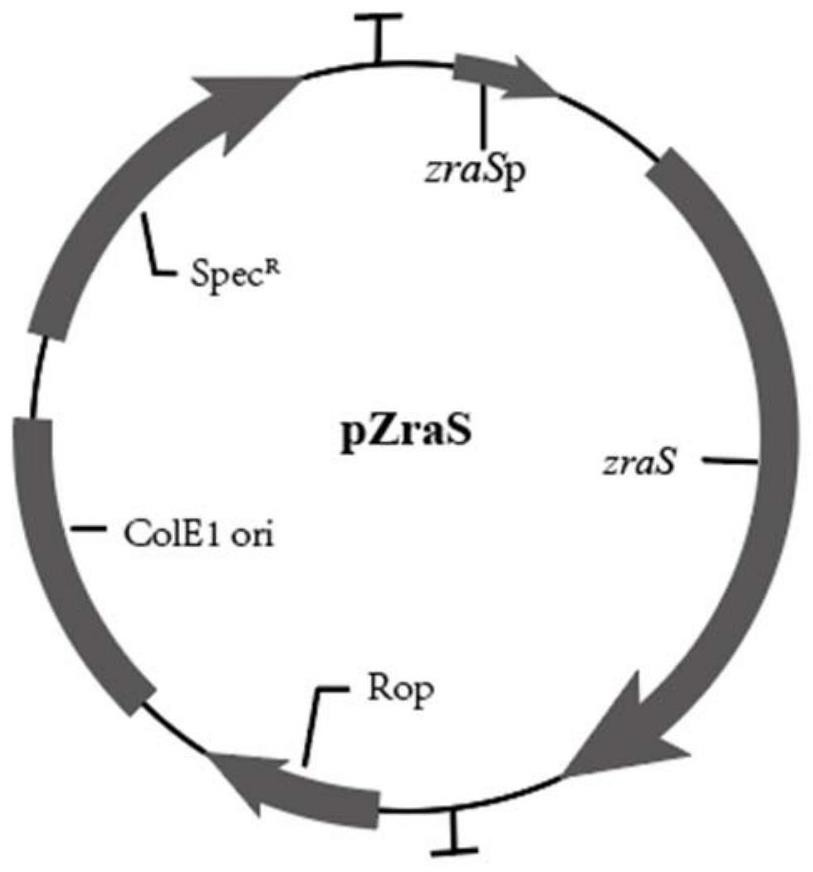
[0076] The present invention also provides a preparation method for the above-mentioned mutant, comprising: constructing SP-ZraS phage; adopting phage-assisted continuous directed evolution (PACE) to alternately evolve the substrate specificity of SP-ZraS phage to lead ions by positive screening and negative screening , to obtain an evolutionary sample; carry out amino acid sequencing analysis on the evolutionary sample to obtain mutation site information; construct the expression vector of the membrane protein ZraS mutant according to the mutation site information, denote as pZraS, and express to obtain the membrane protein ZraS mutant .
[0077] In the present invention, the preparation method specifically includes:
[0078] 1.1 Construction of positive screening helper plasmid and host
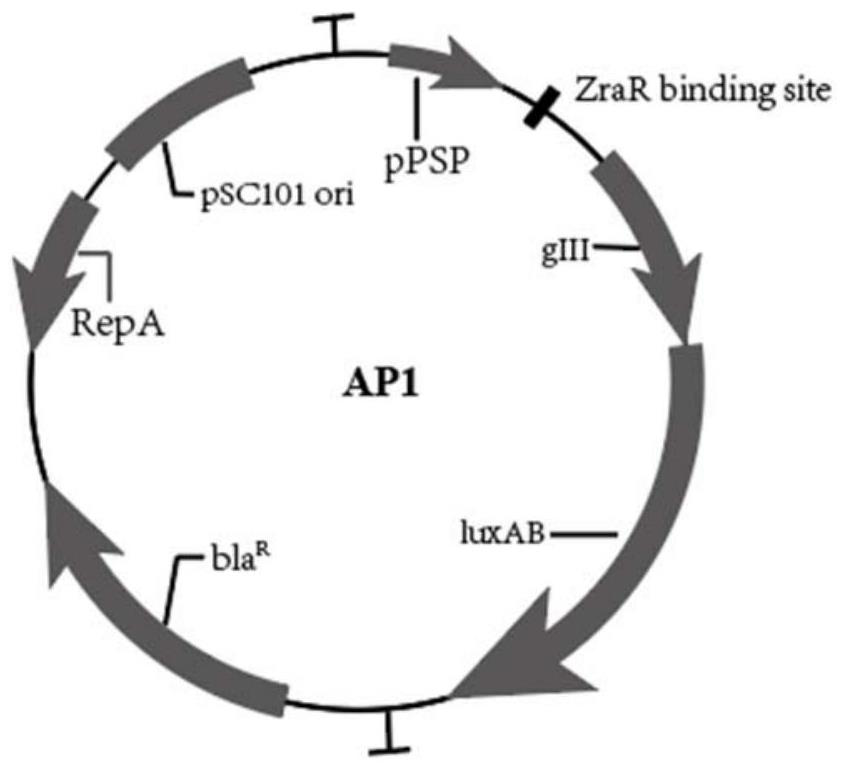
[0079] 1.11 Construction of the gIII protein expression plasmid AP1: the gIII protein is initiated by the promoter psp, and the downstream of the promoter psp is inserted into the recognit...
Embodiment 1
[0102] The ZraS mutation site obtained by directed evolution for lead ion substrate recognition specificity
[0103] 1. Construction of PACE directed evolution helper plasmid
[0104] 1.1 Construction of positive screening helper plasmid and host
[0105] 1) Construction of the gIII protein expression plasmid AP1: the gIII protein is activated by the promoter psp, and the recognition site nucleic acid sequence of the ZraR regulatory protein is inserted downstream of the promoter. AP1 map see image 3 .
[0106] 2) Construction of regulatory protein ZraR and mutagenic gene expression plasmid MP-ZraR: the vector template is mutagenic plasmid MP4, and the ZraR gene sequence is inserted upstream of the arabinose promoter of mutagenic plasmid MP4, and the promoter is J23109. The plasmid can express the regulatory protein ZraR and provide a higher mutation rate under the induction of arabinose during DNA replication. MP-ZraR map see Figure 4 .
[0107] 3) Co-transform E.coli ...
Embodiment 2
[0134] Construction of engineering strains
[0135] 1. Select the mutation sites or combinations obtained in Example 1 to construct different ZraS mutant expression vectors pZraS, and the promoter of ZraS is the endogenous promoter ZraSp.
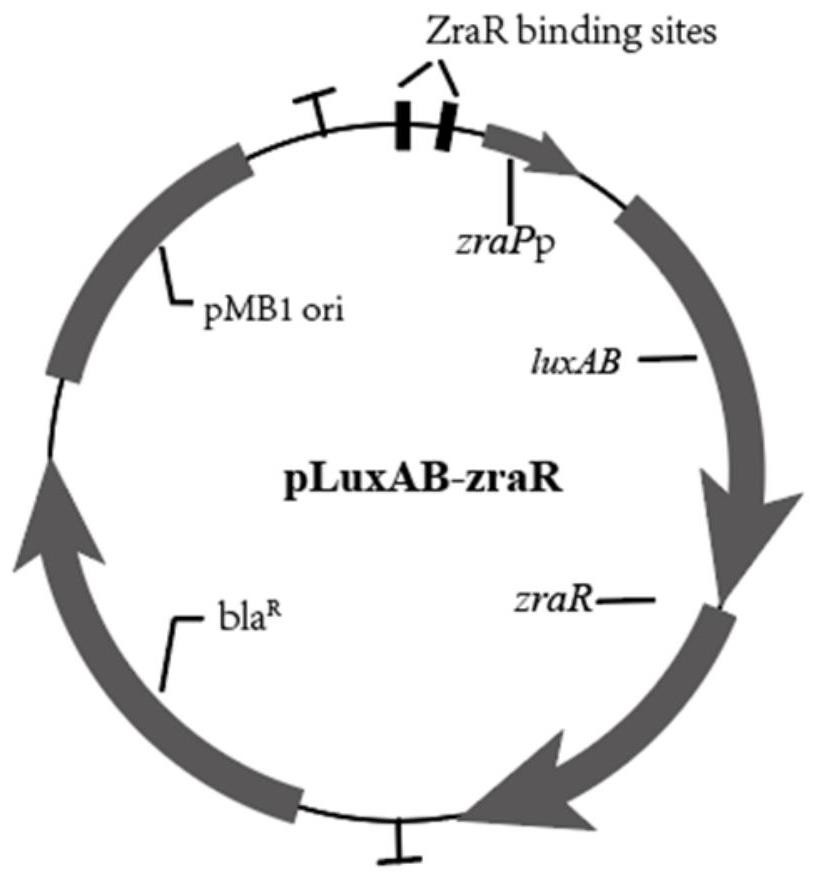
[0136] 2. Construct the expression vector pLuxAB-ZraR of the reporter gene luxAB and the regulatory protein ZraR, see the plasmid map figure 2 . The reporter gene luxAB and the regulatory protein ZraR are expressed in tandem, and are activated by the promoter zraPp, and two binding sites of the regulatory protein ZraR are set upstream of the promoter.
[0137] 3. Different pZraS vectors were co-transformed with pLuxAB-ZraR plasmids to E. coli S1030 competent cells to obtain different recombinant mutant strains.
[0138] The expression host used in the present invention is Escherichia coli S1030 carrying the F plasmid, derived from E.coli DH10B, provided by David R Liu's laboratory, and its genetic information has been reported in relevan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com