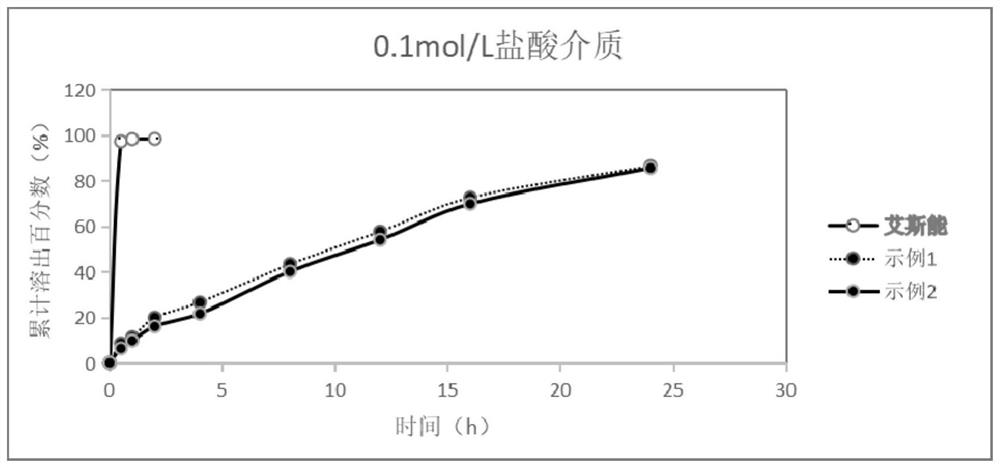
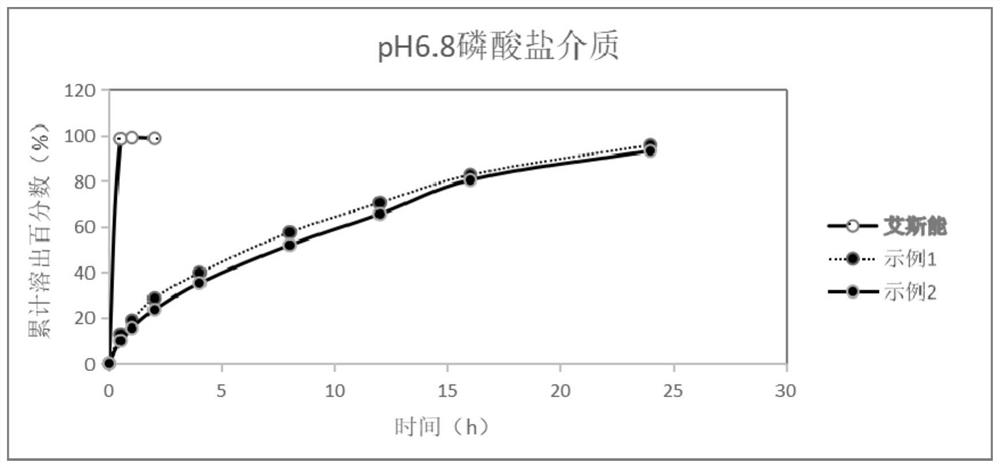
Rivastigmine hydrogen tartrate dry suspension and preparation method thereof
A technology of rivastigmine bitartrate and dry suspension, which is applied in the field of rivastigmine bitartrate dry suspension and its preparation, which can solve the difficulty of increasing the care of family members, the high incidence of adverse reactions, and the inability to take care of themselves, etc. To achieve the effect of simplifying the difficulty of home care, reducing the incidence of adverse reactions, and improving the quality of life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The rivastigmine bitartrate dry suspension disclosed in this example is used in the preparation of 1000 bags, 1.4 g per bag.
[0025] components Dosage (g) Rivastigmine Bitartrate 12 Amberlite IRP 69 100 Ethyl cellulose 30 castor oil 0.9 Polyvinylpyrrolidone K30 0.2 Poloxamer 200 Sodium carboxymethyl cellulose 800 colloidal silica 80 aspartame 100 apple flavor 50
[0026] The preparation method of above-mentioned rivastigmine bitartrate dry suspension is:
[0027] S1. Dissolve the prescribed amount of rivastigmine bitartrate in purified water, add 45-90 μm Amberlite IRP69, and react at 40°C for 10 hours. Filter, rinse the filtrate with purified water, and dry at 40°C to obtain the drug-loaded complex;
[0028] S2. Prepare an ethanol solution containing ethyl cellulose, castor oil, and polyvinylpyrrolidone K30, add the drug-loaded complex in step S1, and stir to disperse. Set the inlet air temp...
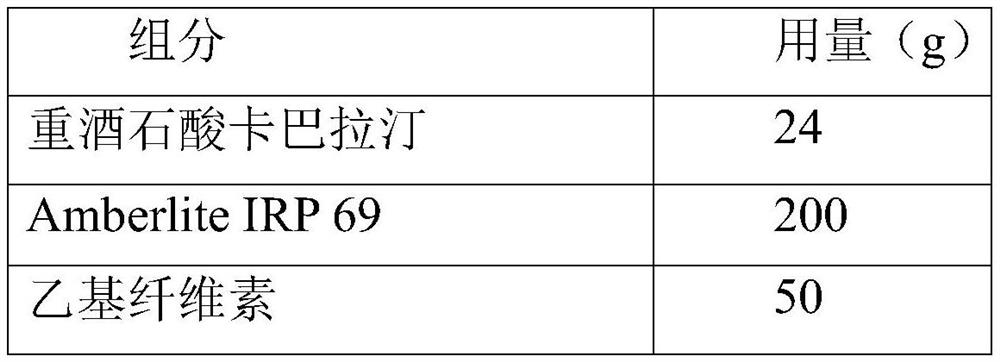
Embodiment 2
[0031] The rivastigmine bitartrate dry suspension disclosed in this example is used in the preparation of 1000 bags, 1.4 g per bag.
[0032]
[0033]
[0034] The preparation method of above-mentioned rivastigmine bitartrate dry suspension is:
[0035] S1. Dissolve the prescribed amount of rivastigmine bitartrate in purified water, add 45-90 μm Amberlite IRP69, and react at 40°C for 12 hours. Filter, rinse the filtrate with purified water, and dry at 40°C to obtain the drug-loaded complex;
[0036] S2. Prepare an ethanol solution containing ethyl cellulose, triethyl citrate, and polyethylene glycol 6000, add the drug-loaded compound in step (1), and stir to disperse. Set the air inlet temperature to 60°C, the centrifugal speed to 15000rpm, and start spray drying.
[0037] S3. Put the spray-dried product in S2 into a three-dimensional mixer together with sodium lauryl sulfate, sodium alginate, aspartame, orange essence, and colloidal silicon dioxide, mix evenly, and pac...
Embodiment 3
[0039] The rivastigmine bitartrate dry suspension disclosed in this example is used in the preparation of 1000 bags, 1.4 g per bag.
[0040]
[0041]
[0042] The preparation method of above-mentioned rivastigmine bitartrate dry suspension is:
[0043] S1. Dissolve the prescribed amount of rivastigmine bitartrate in purified water, add 45-90 μm Amberlite IRP69, and react at 40°C for 12 hours. Filter, rinse the filtrate with purified water, and dry at 40°C to obtain the drug-loaded complex;
[0044] S2. Prepare an ethanol solution containing ethyl cellulose, triethyl citrate, and galactose, add the drug-loaded compound in step (1), and stir to disperse. Set the inlet air temperature to 55°C, the centrifugal speed to 11000rpm, and start spray drying.
[0045] S3. Put the spray-dried product in S2 into a three-dimensional mixer together with poloxamer, sodium carboxymethylcellulose, sucralose, orange essence, and colloidal silicon dioxide, mix evenly, and pack in divided ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com