Amide alkaloid for treating depression and application thereof
A technology of amides and alkaloids, which is applied in the field of medicine, can solve the problems of no compound antidepressant effect, insufficient effective rate, difficulty in obtaining curative effect, etc., achieve good antidepressant activity, reduce immobility time, and have wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
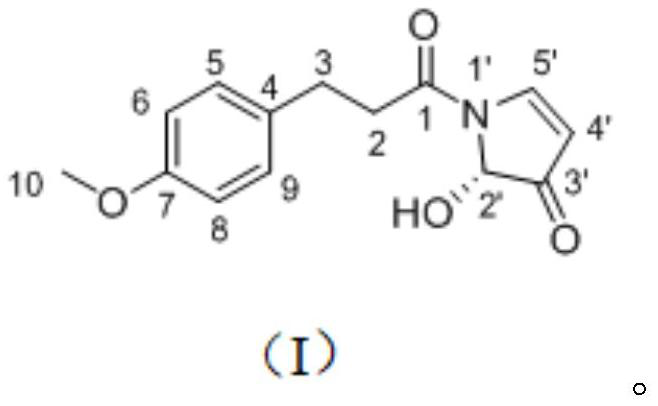
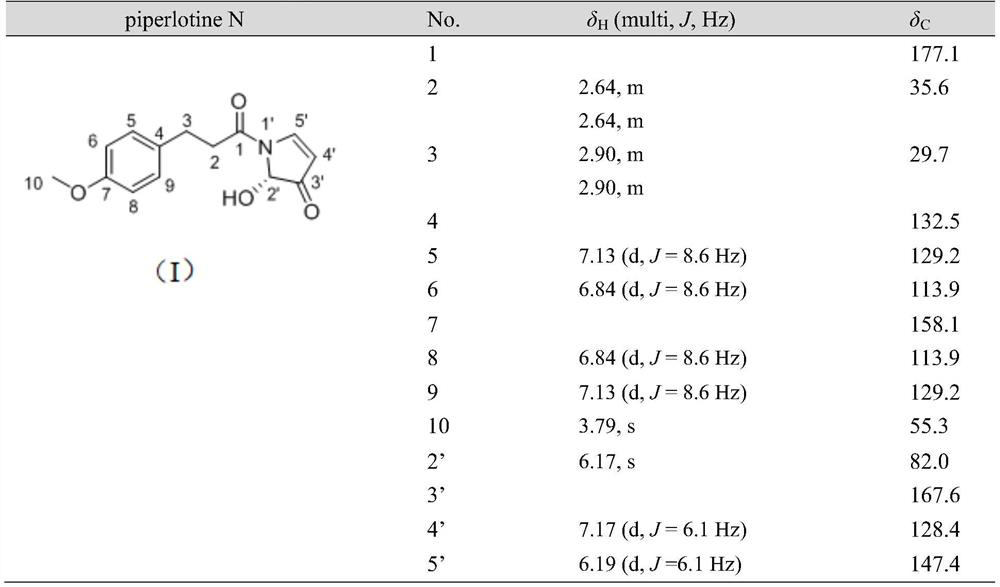
[0023] The preparation of embodiment 1 piperlotine N
[0024]False betel medicinal materials are dried, pulverized and sieved (40 mesh). Heat and reflux extraction with 12 times the volume of 80% ethanol for 3 times, each time for 1.5h, collect the extract and dry it under reduced pressure to obtain the extract of False Konjac. Take betel extract, make a suspension with water, and then carry out liquid-liquid extraction with an equal volume of petroleum ether, and extract 5 times. The extract was collected, concentrated and dried to obtain the petroleum ether part of the extract of Pseudomonas chinensis. Take a sample of the petroleum ether extraction part of Konjac, use 100-200 mesh silica gel (1:1-1.5) to mix the sample, and then separate it by column chromatography silica gel (200-300 mesh), and use petroleum ether-ethyl acetate system as the eluent. The ratio is 20:1, 10:1, 5:1, 2:1, 1:1, and gradient elution is performed. Seven fractions (Frs. 1-7) were combined after ...
Embodiment 2
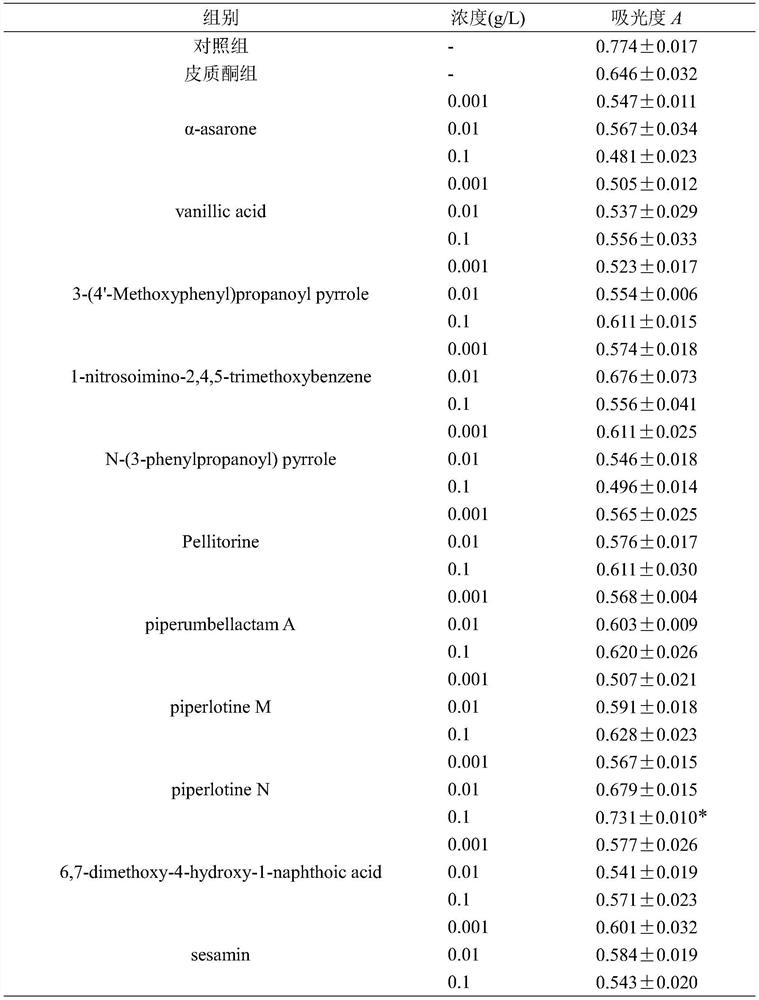
[0028] Example 2: The protective effect of piperlotine N on PC12 cell injury induced by corticosterone
[0029] The CCK-8 method was used to detect the protective effect of the isolated compound on PC12 cell damage induced by high concentration of CORT, so as to detect the antidepressant activity of piperlotine N. Dilute PC12 cells in logarithmic growth phase to 2×10 5 cells / mL, seeded in a 96-well plate, 100 μL per well, placed in 37 °C 5% CO 2 Cultured in an incubator for 24 hours to allow the cells to fully adhere to the wall. Afterwards, different treatments were carried out according to the groups: for the control group, DMEM medium containing 10% fetal bovine serum was added without other treatment; for the CORT injury model group, DMEM medium containing 5×10 CORT was added -4 mol / L complete medium; administration group, dilute the compound stock solution with 1% fetal bovine serum medium to the corresponding concentration and then add, add 5×10 -4 The complete medium...
Embodiment 3
[0033] Example 3: Effect of piperlotine N on immobility time of mice in forced swimming test
[0034] A mouse model of forced swimming test (FST) was established to evaluate the antidepressant activity of piperlotine N. 50 male ICR mice were weighed and recorded, and the mice were randomly divided into 5 groups according to body weight, 10 in each group. The groups were: blank control group (administered with the same volume of distilled water); positive drug group (20 mg / kg of fluoxetine); low, medium and high doses of piperlotine N groups (administered with 10, 20, and 40 mg / kg of piperlotine N, respectively). Continuous intragastric administration for 7 days, after 1 hour of administration on the 7th day, put the mice into a transparent container with a height of 30 cm, a diameter of 15 cm and water (the water depth is 12 cm, to ensure that the mice cannot touch the bottom of the container, and the water temperature is 24-26 ° C). In a cylindrical container, let it swim in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com