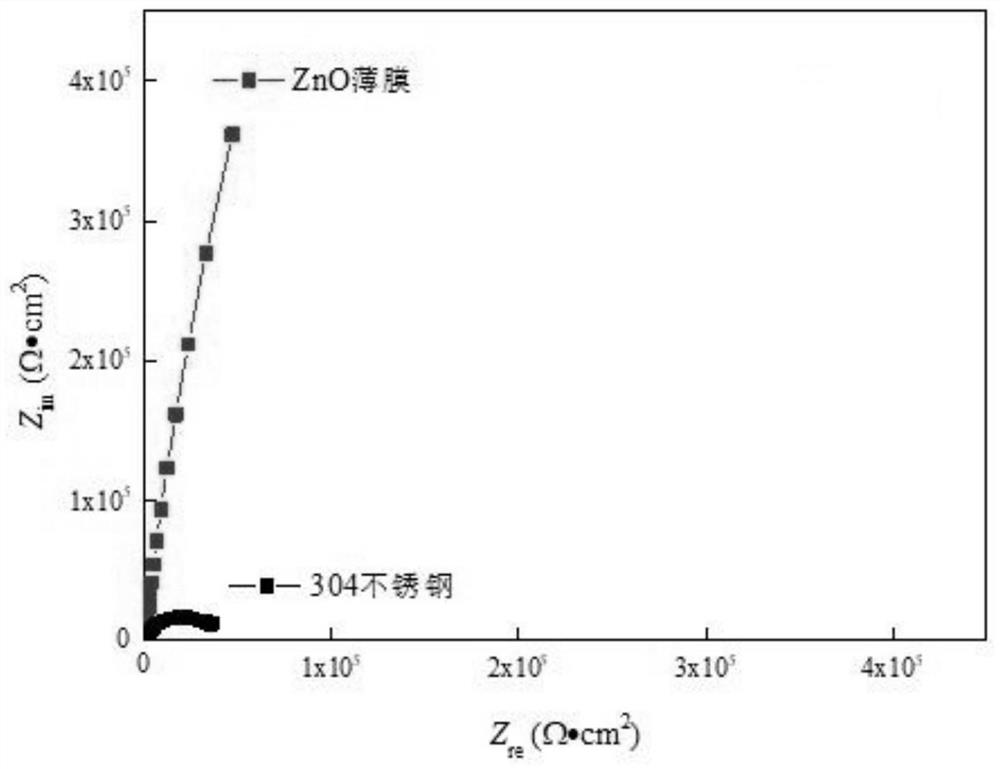
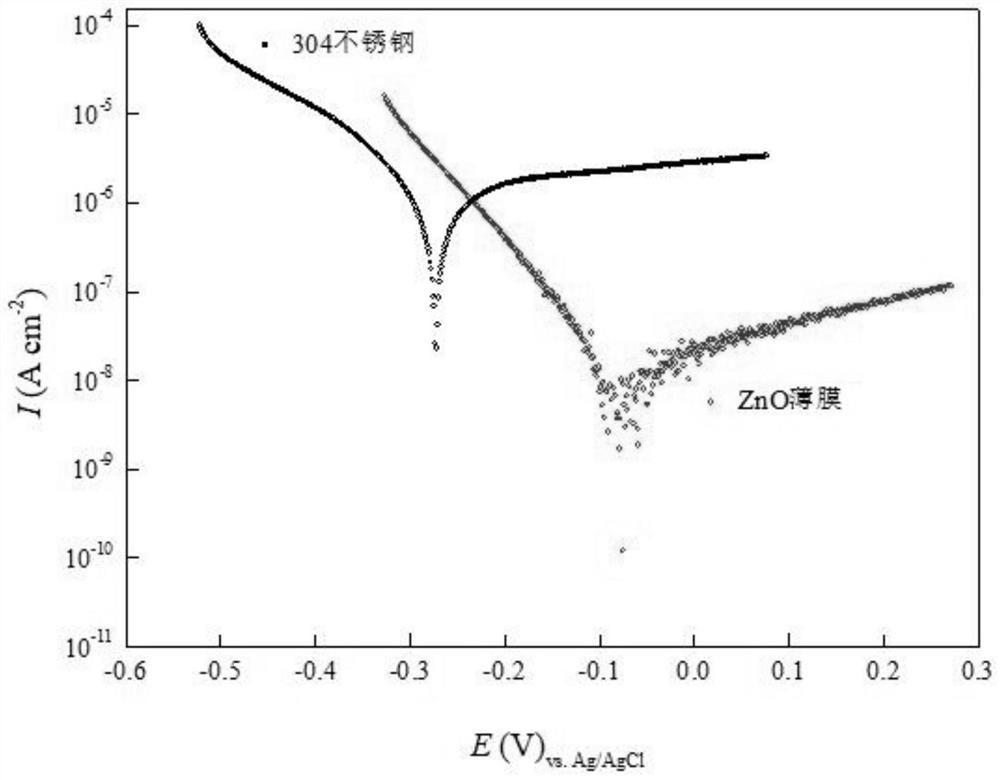
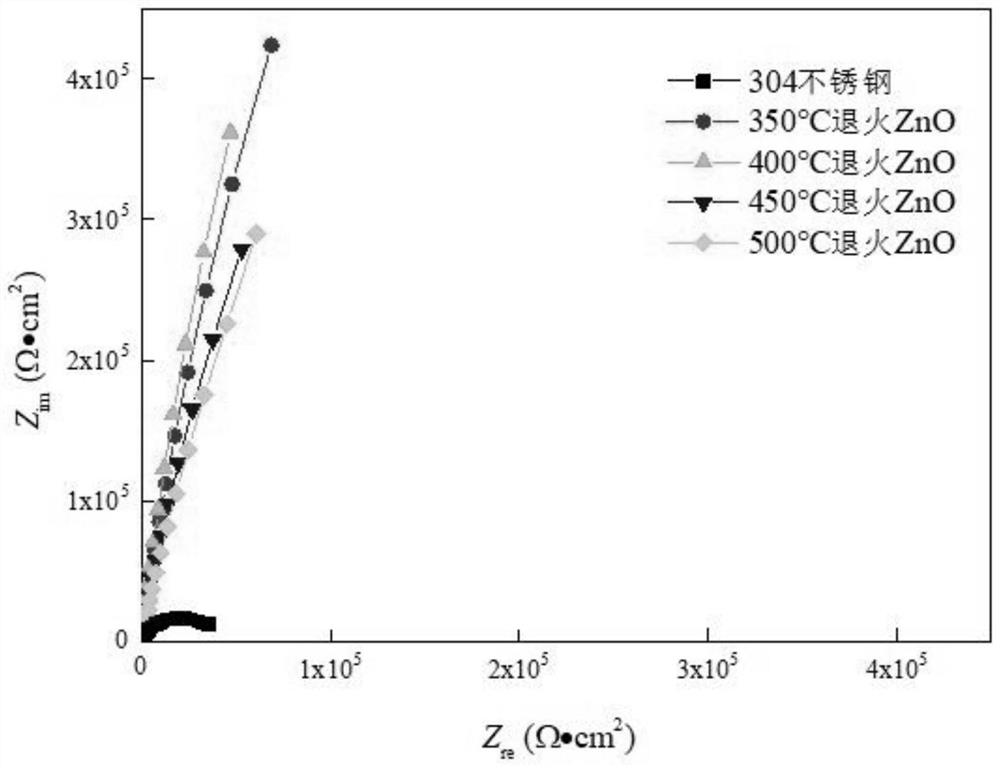
Resistance-variable ZnO corrosion-resistant film and preparation method thereof
A corrosion-resistant film and coating technology, applied in liquid chemical plating, metal material coating process, coating and other directions to achieve the effects of slowing down oxidation, reducing corrosion rate, and slowing down diffusion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Weigh 2.195g of zinc acetate dihydrate and put it in a beaker, add 100ml of ethylene glycol methyl ether. Use a magnetic stirrer to stir, and it can be observed that a turbid solution is formed; after adding ethanolamine in the same molar amount as zinc acetate dihydrate, it can be observed that the turbid solution becomes clear. Stirring was continued for 12h. After aging for more than 24 hours at room temperature, a stable 0.1mol / L zinc acetate sol was obtained.
[0033] (2) 304 stainless steel is used as the base. Use different types of sandpaper to grind in sequence, polish after grinding, clean with deionized water, and ultrasonically in alcohol for 15 minutes, dry and set aside.
[0034] (3) The static spin coating method is used to coat the film, and the rotation speed is controlled at 1000r / min. After the spin coating is completed, put it into an oven at 150° C. for 15 minutes to dry. Repeat the above operation for each layer. A total of 12 layers were s...
Embodiment 2
[0037] (1) Weigh 6.585g of zinc acetate dihydrate and put it in a beaker, add 100ml of ethylene glycol methyl ether. Use a magnetic stirrer to stir, and it can be observed that a turbid solution is formed; after adding ethanolamine in the same molar amount as zinc acetate dihydrate, it can be observed that the turbid solution becomes clear. Stirring was continued for 12h. After aging for more than 24 hours at room temperature, a stable 0.3 mol / L zinc acetate sol was obtained.
[0038] (2) 304 stainless steel is used as the base. Use different types of sandpaper to grind in turn, polish after grinding, clean with deionized water, ultrasonic in alcohol for 18min, dry and set aside.
[0039] (3) Static spin coating method is used to coat the film, and the speed is controlled at 1500r / min. After the spin coating is completed, it is dried in an oven at 130°C for 15 minutes. Repeat the above operation for each layer. A total of 12 layers were spin-coated.
[0040] (4) After the...
Embodiment 3
[0042] (1) Weigh 10.975g of zinc acetate dihydrate and put it in a beaker, add 100ml of ethylene glycol methyl ether. Use a magnetic stirrer to stir, and it can be observed that a turbid solution is formed; after adding ethanolamine in the same molar amount as zinc acetate dihydrate, it can be observed that the turbid solution becomes clear. Stirring was continued for 12h. After aging for more than 24 hours at room temperature, a stable 0.5 mol / L zinc acetate sol was obtained.
[0043] (2) 304 stainless steel is used as the base. Use different types of sandpaper to grind in turn, polish after grinding, clean with deionized water, ultrasonic in alcohol for 17min, dry and set aside.
[0044] (3) Static spin coating method is used to coat the film, and the speed is controlled at 2000r / min. After the spin coating is completed, it is put into an oven at 100° C. for 11 minutes to dry. Repeat the above operation for each layer. A total of 12 layers were spin-coated.
[0045] (4)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com