Intermediate for preparing procaterol hydrochloride and preparation method thereof
A technology for procaterol hydrochloride and intermediates, which is applied in the preparation of intermediates of procaterol hydrochloride and the field of preparation thereof, can solve the problems of not being able to obtain key intermediates and the like, and achieve the effects of simple synthesis process and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
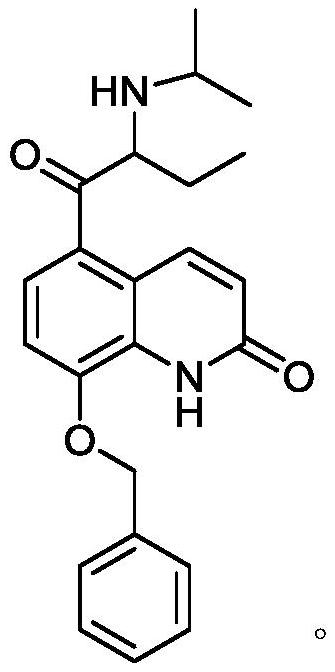
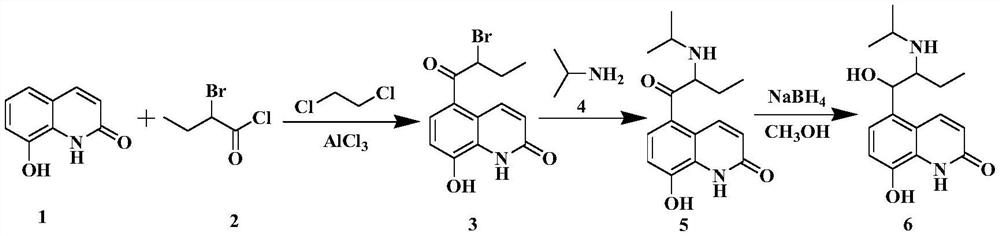
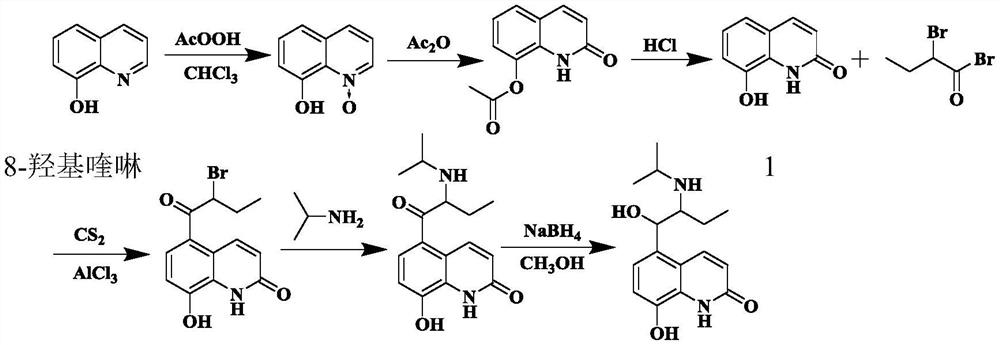
[0078] Example 1 Synthesis of 5-(2-isopropylaminobutyryl)-8-benzyloxyquinolone
[0079] In the reaction flask, add 8-butyryloxyquinolone (54.43g, 0.26mol) shown in formula I, 350.0mL of chloroform and anhydrous aluminum chloride (103.81g, 0.78mol) in turn, stir at room temperature and then add dropwise Butyryl chloride (33.25g, 0.32mol), then heated up to 80°C and reacted for 8h, slowly added the reactant to frozen hydrochloric acid (300.0mL, 5mol / L), filtered, washed with 300mL deionized water, and dried to obtain formula II The shown substance (the mixture of 2A and 2B, the next step reaction is not separated), the yield is 96.0%, and the [M+H] of the two substances by LC-MS at 2.67min and 3.17min + The nucleoplasmic ratio was both 232.1.
[0080] Add water 215.0mL and potassium carbonate (63.45g, 0.46mol) to the reaction flask successively, add acetonitrile 325.0mL and the mixture of 2A and 2B shown in formula II (54.00g, 0.23mol) under stirring, heat to 80 ℃ and drop Ben...
Embodiment 2
[0089] Example 2 Synthesis of 5-(2-bromobutyryl)-8-benzyloxyquinolone:
[0090] Add 8-butyryloxyquinolone shown in formula I (54.43g, 0.26mol), dichloroethane 450.0mL and anhydrous aluminum chloride (109.1g, 0.82mol) in the reaction flask in sequence, and stir evenly at room temperature Afterwards, butyryl chloride (37.41g, 0.36mol) was added dropwise, and then the temperature was raised to 85°C for 9h, and the reactant was slowly added to frozen hydrochloric acid (300.0mL, 5mol / L), filtered, washed with 300mL deionized water, and dried Formula II (mixture of 2A and 2B, proceed to the next reaction without separation) was obtained with a yield of 96.5%.
[0091] Add water 215.0mL and potassium carbonate (63.45g, 0.46mol) in turn to the reaction flask, add acetonitrile 325.0mL and the mixture of 2A and 2B shown in formula II (54.00g, 0.23mol) under stirring, heat to 80°C and add dropwise Benzyl bromide (51.37g, 0.30mol) was reacted for 5h. Add 100.0 mL of water to the reactan...
Embodiment 3
[0117] Example 3 Synthesis of 5-(1-hydroxyl-2-isopropylaminobutyl)-8-benzyloxyquinolone
[0118] 5A (15.0 g, 0.04 mol) obtained in Example 1 and 150 mL of isopropanol were added to the three-neck flask, sodium borohydride (3.78 g, 0.1 mol) was added three times, and the reaction was stirred at 20° C. for 4 h, and 100.0 mL of water was added to quench the reaction. Extracted twice with 200mL ethyl acetate and combined, concentrated to obtain formula VI (6A: 5-(1-hydroxy-2-isopropylaminobutyl)-8-benzyloxyquinolone), the yield was 91.5%, and the HPLC detection purity was greater than 99%. %.
[0119] The H NMR spectrum of 5-(1-hydroxy-2-isopropylaminobutyl)-8-benzyloxyquinolone: 1 H NMR (400MHz, DMSO-d 6 ), δ / ppm: 10.60(s,1H,H1),8.16(d,J=10.0Hz,1H,H3),7.60–7.50(m,2H,H7),7.41–7.34(m,2H,H8) ,7.34–7.27(m,1H,H9),7.22–7.13(m,2H,H4 and H5),6.55(t,J=8.6Hz,1H,H2),5.29(s,2H,H6),4.92( s,1H,H10),1.42(tdd,J=20.6,12.4,8.4Hz,1H,H16),1.29–1.14(m,2H,H13),0.87(t,J=7.6Hz,3H,H14), 0.80-0.67(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com