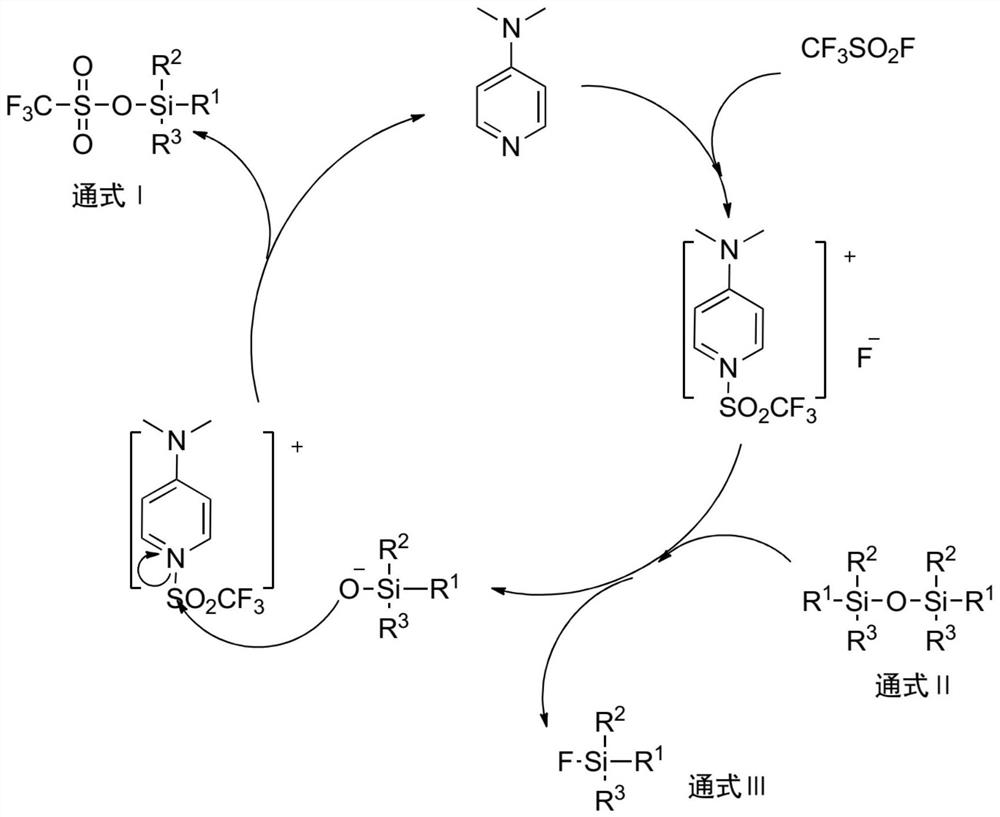
Synthesis method of silyl trifluoromethanesulfonate
A technology of silicon trifluoromethanesulfonate and synthesis method, which is applied in the direction of silicon organic compounds, chemical instruments and methods, compounds of group 4/14 elements of the periodic table, etc., which can solve the problems of expensive raw materials, low activity and reaction, and limited Application and other issues, to achieve the effect of improved reaction safety, simple operation of reaction steps, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
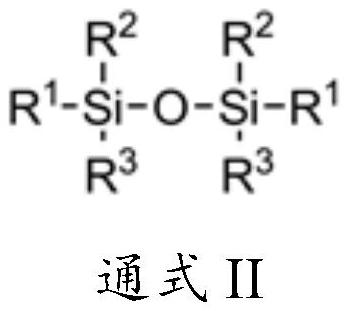
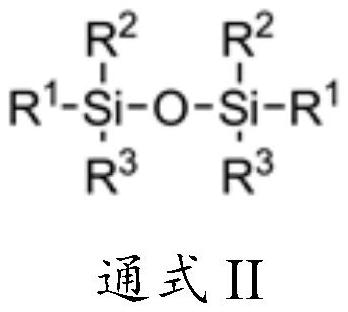
[0040] Add 486g (3mol) of hexamethyldisiloxane and 11.2g (0.1mol) of DMAP into a 2L stainless steel reactor, cool down to -30°C under nitrogen protection, and evacuate the reactor to -0.09MPa. 532 g (3.5 mol) of trifluoromethanesulfonyl fluoride was slowly passed through, and the reaction vessel was closed after the passage was completed, and the temperature was raised to 20° C. for 8 hours. After the reaction, slowly open the outlet valve of the reactor, control the temperature of the condenser at -20°C, and collect the unreacted trifluoromethanesulfonyl fluoride at the temperature of the receiving tank at -30°C. Subsequently, the temperature of the condenser was controlled to 16° C., and the temperature of the receiving tank was 0° C. to collect the by-product trimethylfluorosilane. Increase the vacuum degree of the reactor to -0.08MPa, heat up to 60°C for distillation, collect the fraction at 42-44°C, and obtain 642g of the product trimethylsilyl trifluoromethanesulfonate, ...
Embodiment 2
[0043] Add 493g (2mol) hexaethyldisiloxane and 11.2g (0.1mol) DMAP into a 2L stainless steel reactor, cool down to -10°C under nitrogen protection, and evacuate the reactor to -0.09MPa. 380 g (3.5 mol) of trifluoromethanesulfonyl fluoride was slowly passed through, and the reactor was closed after the passage was completed, and the reaction was carried out at this temperature for 12 hours. After the reaction, slowly open the outlet valve of the reactor, control the temperature of the condenser at -20°C, and collect the unreacted trifluoromethanesulfonyl fluoride at the temperature of the receiving tank at -30°C. Increase the vacuum of the reactor to -0.085MPa, collect the fraction at 55-58°C to obtain triethylfluorosilane; raise the temperature to 90°C, collect the fraction at 85-87°C to obtain the product triethylsilyl trifluoromethanesulfonate 501g, 94.9% yield, 99.2% purity, 0.02% acid content.
[0044]Put 80g of calcium hydroxide (purity 95%) in 1000mL water suspension in...
Embodiment 3
[0046] Add 162 g (1 mol) of hexamethyldisiloxane and 5.6 g (0.05 mol) of DMAP into a 1 L four-necked flask, and replace with nitrogen. The temperature was lowered to -30°C under nitrogen protection, and the reaction bottle was evacuated to -0.09MPa. 182.4 g (1.2 mol) of trifluoromethanesulfonyl fluoride was slowly introduced, and a white solid gradually precipitated in the reaction bottle, and the reaction was carried out at this temperature for 24 hours, and the reaction was stopped. Set up the distillation device, slowly raise the temperature of the reaction bottle to 0°C, control the temperature of the condenser tube at -20~-15°C, and collect the unreacted trifluoromethanesulfonyl fluoride at the temperature of the receiving tank at -35~-30°C. Then raise the temperature of the reaction bottle to 25°C, control the temperature of the condenser tube at 16-20°C, and collect the by-product trimethylfluorosilane at the temperature of the receiving tank at 0°C. Increase the vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com